Dinoflagellates
Mona Hoppenrath and Juan F. Saldarriaga


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Dinoflagellates are common organisms in all types of aquatic ecosystems. Roughly half of the species in the group are photosynthetic (Gaines and Elbrächter 1987), the other half is exclusively heterotrophic and feeds via osmotrophy and phagotrophy. As a consequence, they are prominent members of both the phytoplankton and the zooplankton of marine and freshwater ecosystems. They are also common in benthic environments and in sea ice.

Noctiluca scintillans is a very large marine, planktonic, phagotrophic, athecate dinoflagellate that can cause pinkish red or greenish red tides, that is able to be bioluminescent, and that can contain green eukaryotic endosymbionts (Pedinomonas noctilucae). The shown specimen is from a temperate region (without endosymbionts) but the species can also be found in subtropical and tropical areas. © Mona Hoppenrath
In terms of morphology, dinoflagellates can be as varied and complex as any unicellular eukaryote. Complex organelles found in the group include structures reminiscent of a full-fledged vertebrate eye (but in a unicellular organism that lacks a brain), nematocysts comparable to those of cnidarians, and a bewildering array of plastid types in the photosynthetic forms. Dinoflagellates exist as plasmodia (i.e. multinucleate organisms), biflagellated cells, coccoid stages and even, in one small group, as cell arrays that approach multicellularity.
Genetically, dinoflagellates are also unique. The nucleus of a large majority of dinoflagellates (the so-called dinokaryotes) is so different from other eukaryotic nuclei that it has been given its own name, the dinokaryon. Dinokarya lack nucleosomes, and DNA content is orders of magnitude larger than that of other eukaryotic cells, for example those of humans. These dinokarya divide via a unique form of mitosis. Recent research is starting to show just how unique dinoflagellate genetic systems are. For example, gene products of all dinoflagellate nuclei (not only dinokarya) are processed in a unique way: a spliced leader is trans-spliced to all mRNA molecules. The genomes of plastids and mitochondria of the group are also unique: they are atomized (i.e. the genome is split into very small fragments), and gene content is much, much lower than that of comparable organelles in other organisms.
Approximately 4500 species assigned to more than 550 genera have been described, nearly three quarters of the genera and more than half of the species being fossil. Of the ca. 2000 living species, more than 1700 are marine and about 220 are from freshwater (Taylor et al. 2008). These numbers are sure to grow substantially in the future. Between the years 2000 and 2007 three new dinoflagellate families, 22 new genera, and 87 new species were described (Centre of Excellence for Dinophyte Taxonomy CEDiT). Recent molecular analyses have shown that there are large numbers of undescribed dinoflagellate species in environments like marine picoplankton (e.g. Moreira and López García 2002, Worden 2006) or as symbionts (‘zooxanthellae’) in many types of protists and invertebrates like corals (Coffroth and Santos 2005).
Practical Significance
Dinoflagellates are perhaps best known as causers of harmful algal blooms (webpages about this topic: ISSHA, WHOI, IOC). About 75-80% of toxic phytoplankton species are dinoflagellates (Cembella 2003), and they cause “red tides” that often kill fish and/or shellfish either directly, because of toxin production, or because of effects caused by large numbers of cells that clog animal gills, deplete oxygen, etc. (Smayda 1997). Dinoflagellate toxins are among the most potent biotoxins known. They often accumulate in shellfish or fish, and when these are eaten by humans they cause diseases like paralytic shellfish poisoning (PSP), neurotoxic shellfish poisoning (NSP), diarrheic shellfish poisoning (DSP) and ciguatera (Lehane and Lewis 2000). They also have been linked to major human health concerns, especially in estuarine environments (Pfiesteria). Some syndinians, notably Hematodinium, are parasites of economically-significant crustacean species.
The main ecological significance of dinoflagellates lies elsewhere, though. They are second only to diatoms as marine primary producers. As phagotrophic organisms they are also important components of the microbial loop in the oceans and help channel significant amounts of energy into planktonic food webs. As zooxanthellae, dinoflagellates have a pivotal role in the biology of reef-building corals.
Characteristics
Typically, dinoflagellate motile cells are biflagellated unicells, between 10 and 100 µm in length (the extreme range is 2 to 2000 µm). Even for single cells like these you need to understand the orientation of a motile dinoflagellate cell to describe the morphology properly.
Flagella
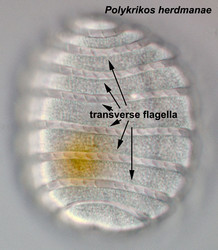
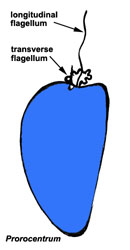
Motile cells possess two dissimilar flagella arising from the ventral cell side = dinokont flagellation (Fig. 7). They have a ribbon-like transverse flagellum with multiple waves that beats to the cell’s left, and a more conventional one, the longitudinal flagellum, that beats posteriorly (Figs 1, 2; Taylor 1975, Leblond and Taylor 1976, Gaines and Taylor 1985, Fensome et al. 1993). The transverse flagellum is a wavy ribbon (Figs 3-6) in which only the outer edge undulates from base to tip, due to the action of the axoneme which runs along it. The axonemal edge has simple hairs that can be of varying length. The flagellar movement produces forward propulsion and also a turning force. The longitudinal flagellum is relatively conventional in appearance, with few or no hairs. It beats with only one or two periods to its wave. The flagella lie in surface grooves: the transverse one in the cingulum and the longitudinal one in the sulcus, although its distal portion projects freely behind the cell. In dinoflagellate species with desmokont flagellation (e.g., Prorocentrum) the two flagella are differentiated as in dinokonts, but they are not associated with grooves (Fig. 8).
Nucleus
The nucleus of most dinoflagellates, the dinokaryon, is unique (Fig. 9). Dinokarya lack nucleosomes (Rizzo 1991), and the ratio of basic proteins to DNA in them is much lower than in any other eukaryotes. They contain very high amounts of DNA per cell: 3,000-215,000 Mbp (humans have 2,900 Mbp DNA/cell). Chromosomes appear fibrillar because they remain continuously condensed during both interphase and mitosis, the 3–6 nm fibrils being packed in a highly ordered state (up to six levels of coiling) consisting of arches and whorls (Dodge 1966, Spector et al. 1981). A prominent nucleolus is also persistent. Most basic nuclear proteins in dinokarya are not histones. However, recent data suggests that histones do exist in dinoflagellate nuclei, albeit in very low quantities. In some dinoflagellates (Noctiluca, Blastodinium) chromosomes do decondense (to a degree) during interphase. At some stage in their life cycle - usually gametogenesis - the chromosomes reassume a typical dinokaryotic appearance.
Dinoflagellate mitosis is also unusual (dinomitosis). It is closed (i.e. the nuclear envelope persists during mitosis), and the mitotic spindle is extranuclear. Spindle microtubules pass through furrows and tunnels that form in the nucleus at prophase (Dodge 1987 and references therein). There are no obvious spindle pole bodies other than concentric aggregations of golgi bodies (“archoplasmic spheres”). Some microtubules contact the nuclear envelope, lining the tunnels at points where the chromosomes also contact. The chromosomes usually have differentiated, dense regions inserted into the envelope. The anomalous genus Oxyrrhis has closed mitosis with an internal spindle.
Amphiesma
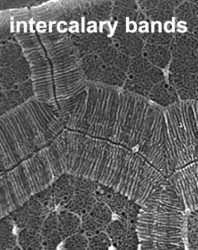
The organization of the outer cortical region of dinoflagellates is distinctive. This entire structural complex, regardless of the presence or absence of cellulose plates, is the amphiesma (Morrill and Loeblich 1983, Netzel and Dürr 1984). A single layer of vesicles is usually present beneath the cell membrane, the alveolae (= amphiesmal vesicles, Fig. 10). The vesicles themselves play a structural role. Cells can be athecate (naked) or thecate (posses a wall). In athecate species the vesicles are either empty or contain amorphous material. In walled dinoflagellates, close-fitting cellulosic plates - which together form the theca - are contained within the alveolae, one per vesicle (Fig. 11 coming soon). The thecal plates usually fit tightly together (Fig. 12), with the margins often overlapping (imbrication pattern). The boundaries of the plates are the sutures. Cell growth occurs by the addition of wall material along some of the margins of the thecal plates. These growth zones (intercalary bands) are often striated (Fig. 13). The patterns formed by the thecal plates (see Tabulation) are of critical importance in taxonomy.


Fig. 10. Transmission electron micrograph (TEM) of alveolar vesicles in cross section. ©
Some species have a continuous fibrous layer under the alveolae, the dino-pellicle (Fig. 14 coming soon), made of cellulose, usually with sporopollenin added to varying degrees. In so-called pelliculate dinoflagellates it may form the principal strengthening layer of the amphiesma. In thecate genera it is usually present under the theca and forms the wall of temporary cysts that can be formed rapidly and asexually by the shedding of the theca (ecdysis). Microtubules are usually present under the vesicles of both thecate and athecate forms, presumably adding some strength to the latter and aiding in morphogenesis.
Vacuoles and Pusules
A large part of the volume of dinoflagellate cells is taken up by a system of vacuoles collectively known as the vacuome. In addition to them there are usually two specialized vacuoles that arise from ducts that open at the flagellar bases, the pusules (Fig. 15). Different morphotypes of pusules are known (Dodge 1972). A sac pusule can occupy a third or more of an episome, and a collecting pusule (Fig. 16) resembles a cluster of grapes. Each has evaginations, which can be highly elaborate, running close to the vacuome membrane. Although they resemble osmoregulatory vacuoles, they do not behave like them. Their function is still unknown. They are most developed in heterotrophic marine species (Fig. 15).
Mitochondria
Dinoflagellate mitochondria have tubular cristae (Fig. 17) constricted at the base and arising from the inner membrane. Mitochondrial genomes in dinoflagellates encode much fewer genes than those of any other eukaryotes except for their apicomplexan relatives. To date, only three complete mitochondrial-encoded protein genes have been found in dinoflagellates: cytochrome oxidase 1 (cox1), cytochrome oxidase 3 (cox3), and cytochrome b (cob). In addition, there are pieces of ribosomal RNA genes (Nash et al. 2007, Slamovits et al. 2007). Coding sequences of mitochondrial genes do not map to a single chromosome, the genetic material in dinoflagellate mitochondria seems to be atomized into many small chromosomes, each one with one or a few genes or gene fragments. There is also evidence of extensive post-transcriptional RNA editing (Lin et al. 2002, Zhang and Lin 2005).


Fig. 17. TEM details of dinoflagellate mitochondria with tubular christae. Image take by Brian Leander. © Mona Hoppenrath
Plastids and Pyrenoids
Roughly half of the dinoflagellate species are photosynthetic, but completely autotrophic species are very rare (Schnepf and Elbrächter 1992). Photosynthetic dinoflagellates are generally mixotrophic and rely on a combination of photosynthesis and heterotrophic nutrition. The diversity in chloroplast-types that exists within dinoflagellates is unparalleled within any group of eukaryotes (Schnepf and Elbraechter 1999).
The main type of pastid in the group, the so-called peridinin plastid, is present in a large majority of photosynthetic dinoflagellates. It contains triple-membraned (sometimes double-membraned) envelopes, thylakoids usually in groups of unappressed threes (Fig. 18) and various types of pyrenoids (Schnepf and Elbrächter 1999). No girdle lamellae are present. Photosynthetic pigments include chlorophylls a and c2 as well as peridinin (a type of carotenoid only found in dinoflagellates), b-carotene, small amounts of diadinoxanthin and dinoxanthin (Jeffrey et al. 1975). The usual storage products in dinoflagellates are starch, produced exterior to the plastid, and oils. DNA-containing areas in peridinin plastids may be single or multiple, sometimes in prominent “nucleoid-like” regions (Dodge 1973).

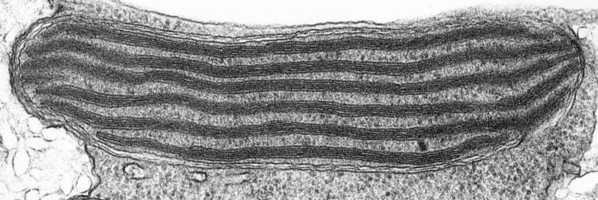
Fig. 18. TEM of a typical peridinin dinoflagellate chloroplast. Image copyright ©
The genetic systems of peridinin plastids, just like those of dinoflagellate nuclei and mitochondria, are completely unique. Peridinin-containing plastids appear to contain genes that exist as minicircles with usually one gene per circle (but two or even three genes in a circle also exist) flanked by a variety of non-coding sequences (Zhang et al. 1999, review in Howe et al. 2008). The absolute number of genes coded in the dinoflagellate peridinin plastids also seems to be much lower than in other algae: while the plastid of cryptomonads, diatoms and other photosynthetic chromalveolates codes for around 165-185 genes, no more than 16 genes have ever been found in any dinoflagellate peridinin plastid (Green 2004, Nisbet et al. 2004). Some of the missing genes appear to have been moved to the nucleus of the organisms involved (e.g. Hackett et al. 2004, Bachvaroff et al. 2004), but there are still a number of them that are missing altogether. Interestingly, peridinin plastids have a bacterial type of rubisco (evidently a lateral gene transfer) that has a much lower specificity for CO2 over O2 when compared to the more common ‘eukaryotic’ rubisco found in other algae (Whitney et al. 1995, Morse et al. 1995).
Nevertheless, many photosynthetic dinoflagellates have photosynthetic organelles that differ in practically all respects from the typical peridinin plastids and also from each other. When speaking about these organelles, it is not always easy to distinguish between true plastids (i.e. organelles that include proteins encoded in their host’s nucleus) and recent and/or temporary endosymbioses of either complete organisms, or fragments of organisms (plastids) that have been recruited to perform photosynthesis. Temporary plastids that are taken from prey and need to be replenished regularly are called kleptochloroplasts (‘stolen’ chloroplasts), they are often derived from cryptomonads. Endosymbioses between otherwise non-photosynthetic dinoflagellates and complete algal cells (not just their plastids) are also known (e.g. Noctiluca and its endosymbionts, members of the prasinophycean genus Pedinomonas), and in some cases these symbioses become so close that the host cell is only known in combination with its endosymbiont. These sorts of relationships are thought to have given rise to new types of plastids. Also endocytobiosis of several eukaryotic cells per dinoflagellate cell is known from Podolampas bipes (Schweikert and Elbrächter 2004).
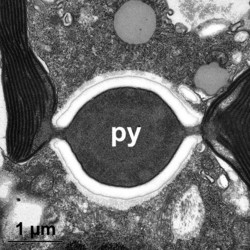
Given this degree of plastid diversity, it is not surprising that there are many types of pyrenoid in dinoflagellates. However, even within peridinin-plastids the structure of pyrenoids varies greatly (Figs 19-21). Some species lack pyrenoids altogether. Otherwise, pyrenoids can be internal or terminal, and may or may not be traversed by thylakoids or associated with starch. Some pyrenoids are stalked or even multiple stalked. Five main types were distinguished by Dodge and Crawford (1971).
Feeding Structures
Heterotrophic taxa can be either free-living or parasitic, and they rely on both osmotrophy and phagotrophy. Prey capture mechanisms in phagotrophic forms vary greatly. Direct phagocytosis occurs in several species of athecate dinoflagellates. A distinct cell mouth (cytostome) is then present (e.g., Noctiluca, Oxyrrhis, Gyrodinium), and it is capable of distending greatly as large prey organisms are eaten. Species of Gyrodinium or Noctiluca are capable of engulfing whole diatom chains, whole copepod eggs, and other relatively large objects.
Peduncle: Myzocytosis is a different form of feeding that involves piercing the prey’s cell membrane with a special organelle, the peduncle, and somehow ‘sucking’ the prey cell’s contents as if through a straw (e.g. in Paulsenella, Pfiesteria, see Schnepf and Elbrächter 1992).
Pallium: Thecate dinoflagellates can’t expand in volume the same way that athecate ones can, and thus are unable to ingest large prey items directly. Instead they extend a delicate, pseudopodial “feeding veil”, the pallium (video clips of pallium feeding), with which they surround portions of diatom chains or other large prey. Digestive enzymes are secreted by the pallium, and digestion then occurs “extracellularly”. The veil is retracted afterwards (Gaines and Taylor 1984, Jacobson and Anderson 1992).
Extrusomes
Dinoflagellates, like other alveolates, often contain a wide array of organelles whose job is to secrete material to the exterior. We distinguish the following types:
- Trichocysts (Figs 22, 23): The most common type of extrusome, of almost universal occurrence, trichocysts are rod-shaped when mature. They usually lie in the amphiesma, perpendicular to the cell membrane. The shaft is a paracrystalline, proteinaceous rod a few micrometers in length, rectangular in cross-section. It is enclosed within a membranous sac, and there is a sheathing material between the rod and the membrane (Livolant, 1983a, b). The tip of the sac is in contact with the cell membrane, passing through the amphiesmal vesicles (and thecal plates, if present). Their function is unknown, but is assumed to be defensive, excretory, or both.
- Mucocysts (Fig. 24 coming soon): Extrusomes that produce mucoid material. Two types are known, simple flask-shaped vesicles with granular contents or vermiform vesicles with diffuse fibrous material (Hoppenrath and Leander 2008).
- Nematocysts (Fig. 25): Complex organelles found in warnowiids and polykrikoids that resemble the stinging organelles (cnidocysts) of cnidarians (Westfall et al. 1983). Nematocysts are larger than trichocysts and can reach 20 µm in length. They are conical, fluid-filled vesicles with a capitate blunt end. Most of the body consists of a large posterior chamber, supported by longitudinal ribs in Nematodinium, from which a smaller anterior chamber is isolated. The whole structure is capped by a lid-like operculum. A sharp stylet in the anterior chamber is connected to a tubular filament in the posterior chamber. In Polykrikos it is coiled much like those in cnidarians and the nematocysts fire by inversion, the stylet driving through the operculum. In Polykrikos two other structures are invariably associated with the nematocysts: a taeniocyst (Fig. 25), which resembles a trichocyst in that it is a solid rod but with more elaborate differentiation, and a chute, which appears to act as a safe conduit to the exterior when the complex discharges (Westfall et al. 1983, Hoppenrath and Leander 2007a,b).
Eyespots
No protist group displays as many eyespot types as dinoflagellates (Hansen et al. 2007b). Four Dinoflagellate Eyespot Types have been distinguished, all of them situated in the sulcal area close to the flagellar roots where they are likely to be shadowed by the proximal part of the longitudinal flagellum.
Complex Organelles
One group, the warnowiids, has developed a variety of complex organelles not found in other dinoflagellates.
Ocelloids: The ocelloid found in warnowiid genera is a complex organelle showing extraordinary resemblances to metazoan eyes, but at a subcellular level. It is entirely comparable to vertebrate eyes. Ocelloid types of different complexity and location in the cell are known (video clip of an ocelloid). Ocelloids consist of three primary components: a lens-like, refractile hyalosome, an ocular chamber, and a darkly pigmented melanosome (Greuet 1978). Microtubules are able to change the shape of the lens, and it appears that it can focus light on the surface of the retinoid (Francis 1967), although how this information is used is unclear.
Piston: This posterior tentacle is covered by many mitochondrial papillae, has a remarkable capacity for contraction, and is highly differentiated - mitochondrial cuff, myonematic arcs, skeletal bands, axial canal and its extension in the cellular body, compensatory sac (Greuet 1987). It is only present in two warnowiid genera, Erythropsidinium and Greuetodinium. Erythropsidinium seems to move due to the pistion (video clip of Erythropsidinium moving).
Nematocysts: Extrusomes of different complexity, see above.
Scintillons
A special feature of ca. 18 dinoflagellate genera (Poupin et al. 1999), scintillons are individual cytoplasmic bodies (ca. 0.5 µm in diameter) distributed mainly in the cortical region of the cell, outpockets of the main cell vacuole. They contain luciferase, the main enzyme involved in dinoflagellate bioluminescence, and luciferin, a chlorophyll-derived tetrapyrrole ring that acts as the substrate to the light-producing reaction. The luminescence occurs as a brief (0.1 sec) blue flash (max 476 nm) when stimulated, usually by mechanical disturbance. Dinoflagellates account for much of the bioluminescence in the world’s oceans. Dinoflagellate bioluminescence is controlled by circadian rhythms and only occurs at night (e.g., Knaust et al. 1998). Luminescent and nonluminescent strains can occur in the same species.
Life Cycle
Dinoflagellates generally have zygotic life cycles (i.e. with post-zygotic meiosis), and motile life stages are normally haploid. Population growth normally occurs through asexual division. If environmental conditions trigger sexuality, gametes are formed. Clearly established sexual fusion has been seen only in a few species, but it is probably widespread (gametes resemble regular motile cells, and fusion occurs at night in photosynthetic species). Syngamy may involve equal (isogamy) or unequal (anisogamy) motile gametes. Both homothallism (gamete fusion in clonal strains) and heterothallism (no fusion in clonal strains) are known. Also complex heterothallism with more than two sexual types is known for some Alexandrium species (Figueroa et al. 2007).
The product of gamete fusion is a planozygote, which may remain motile for hours or a few days. Eventually a nonmotile thick-walled resting cyst (hypnozygote) is formed. Excystment occurs after a varying length of time of dormancy. Meiosis is heralded by a peculiar churning and rotation of the nucleus, a process termed nuclear cyclosis associated with the pairing of homologous chromosomes (video clip of nuclear cyclosis). Meiosis may precede or follow excystment and is normally accomplished in two conventional, successive divisions. Dinoflagellate life cycles can be much more complicated than this (e.g. Figueroa et al. 2006 found multiple routes of sexuality in Alexandrium taylori). The mobile zygotes can follow three different routes: (1) direct division by desmoschisis, (2) short-term encystment (temporary, ecdysal cysts), and (3) long-term encystment (resting cysts).
See Dinoflagellate Life Cycles for a more detailed description.
Circadian Rhythms
In a number of species many cellular phenomena are rhythmic, exhibiting daily (circadian) differences. Processes such as bioluminescence, photosynthesis, cell division, and motility have all been studied intensively, especially in Lingulodinium polyedrum (Sweeney 1987, Akimoto et al. 2004). A key feature of the circadian (about one day) control is that the mechanism responsible is endogenous, not directly dependent upon the light-dark cycles, which, however, serve to confer phase to the system (Johnson and Hastings 1986).
Ecology
Dinoflagellates can be found in most aquatic environments, both freshwater and marine, and in intrazoic habitats.
Plankton
The greatest concentrations of dinoflagellates in the phytoplankton (107–108/l) occur in temperate coastal waters subject to transient periods of vertical stability (Taylor et al. 2008). In temperate coastal and fresh waters, dinoflagellates usually bloom in mid- to late summer when sunshine and vertical stability allow strong aggregations to develop at vertical and/or horizontal discontinuities. They are most abundant at the end of blooms of their prey organisms. In tropical waters, as well as in nutrient-poor temperate regions (e.g. oceanic gyres), all types of phytoplankton are impoverished, but under some conditions dinoflagellates can be relatively numerous. Mixed coastal temperate waters and polar waters are more likely to be dominated by diatoms than by dinoflagellates. Heterotrophic species (microzooplankton) depend on the presence of their food for nutrition, they are most numerous when their food is available.
Benthos
Benthic dinoflagellates are common inhabitants of sediments (especially sand). Photosynthetic forms can bloom, and several species may discolor marine tidal sand flats. Crypthecodinium cohnii and Oxyrrhis marina are often associated with seaweeds, and the latter also forms intense pink tide-pool blooms. Toxic species are common on tropical, bushy seaweeds.
Intrazoic symbioses
Most zooxanthellae are dinoflagellates. The association between dinoflagellates and reef-building corals is widely known, but dinoflagellate endosymbionts inhabit a great number of other invertebrates and protists, for example many sea anemones, jellyfish, nudibranchs, the giant clam Tridacna, as well as several species of radiolarians and foraminiferans (e.g., Trench 1997). Many extant dinoflagellates are parasites (here defined as organisms that eat their prey from the inside, i.e. endoparasites, or that remain attached to their prey for longer periods of time, i.e. ectoparasites). They can parasitize animal or protist hosts. Protoodinium, Crepidoodinium, Piscinoodinium and Blastodinium retain their plastids while feeding on their zooplanktonic or fish hosts. In most parasitic dinoflagellates the infective stage resembles a typical motile dinoflagellate cell.
Culture Availability
It is as a rule much easier to culture photosynthetic dinoflagellates than their non-photosynthetic relatives (Oxyrrhis and Crypthecodinium are examples of easily-growable non-photosynthetic species). As a consequence there is a strong bias in culture collections towards photosynthetic forms, especially toxic and bioluminescent species. The following are good sources of dinoflagellate cultures:
- Provasoli-Guillard National Center for Culture of Marine Phytoplankton (CCMP, Boothbay Harbor, Maine, USA)
- Canadian Center for the Culture of Microorganisms (CCCM, Vancouver, Canada)
- CSIRO Collection of Living Microalgae (CSIRO, Hobart, Tasmania, Australia)
- Cawthron Institute Culture Collection of Micro-algae (CICCM, Nelson, New Zealand)
- Culture Collection of Algae and Protozoa (CCAP, Oban, UK)
- Microbial Culture Collection at the National Institute for Environmental Studies (MCC-NIES, Tsukuba, Japan)
Relationships of Dinoflagellates to Other Organisms
The closest relatives of dinoflagellates are apicomplexans and ciliates. These three eukaryotic clades, together with the paraphyletic group that includes their ancestors, the protalveolates, form the so-called Alveolates (Cavalier-Smith 1991), one of the best-supported groupings that have emerged from the analysis of molecular phylogenetic data in eukaryotes (e.g. Fast et al. 2002, Cavalier-Smith and Chao 2004, and many others). Morphological data also strongly supports this clade (e.g. Taylor 2004). The closest relatives of alveolates are the heterokonts (also called stramenopiles). The relationship between alveolates and heterokonts is also very well supported with molecular data (e.g. Fast et al. 2001, Harper and Keeling 2003, Hackett et al. 2004a). Alveolates, heterokonts and a clade composed of cryptomonads and haptophytes have been proposed to constitute the so-called chromalveolates, one of the supergroups of eukaryotic diversity.
If the chromalveolate hypothesis is true (and if the dinoflagellate peridinin plastid is a vertical descendant of the original chromalveolate plastid), then the ancestor of all dinoflagellates was photosynthetic, and it contained the same type of plastids as the ancestor of all apicomplexans. The close relationship between dinoflagellates and apicomplexans, and the abundance of parasitic groups branching from the base of the dinoflagellate lineage (syndinians) argue furthermore for a parasitic (or perhaps mutualistic?) ancestor for the whole group. The recent discovery of a photosynthetic endosymbiont of corals, Chromera, with apicomplexan phylogenetic affinities strongly supports these two views (Moore et al. 2008). It was shown in that study that the apicomplexan apicoplast (a plastid remnant in that group) was derived from a red alga in the same endosymbiosis event that gave rise to the dinoflagellate peridinin plastid (Moore et al. 2008, Keeling 2008). This event could possibly have been the original chromalveolate endosymbiosis. Recent data also suggest that the nuclei of organisms from at least some of the early, non-photosynthetic branches of the dinoflagellate lineage, e.g. Perkinsus (Stelter et al. 2007, Matsuzaki et al. 2008) and Oxyrrhis (Slamovits and Keeling 2008) contain genes of a plastidial origin.
Whether the chromalveolate hypothesis turns out to be correct or not, at least the dinokaryotic non-photosynthetic dinoflagellates seem to have had photosynthetic ancestors: photosynthetic and non-photosynthetic forms always make mixed groups in phylogenetic trees, and since the typical dinoflagellate peridinin plastid is exceedingly unlikely to have originated more than once, a repeated loss of photosynthetic ability in the non-photosynthetic groups is a virtual certainty (Saldarriaga et al. 2001, Sánchez-Puerta et al. 2007). The presence of cryptic plastids in ostensibly non-photosyntetic forms (e.g. Sparmann et al. 2008) is significant in this regard. In some lineages, the peridinin-plastid was not only lost, but also replaced by either true plastids or plastid-like organelles with very different characteristics (see Plastids and Pyrenoids). The molecular mechanisms that enable this ‘plastidial promiscuity’ in dinoflagellates are poorly understood, but they are likely to involve signal sequences that tag nuclear-encoded proteins to peridinin-containing plastids somehow being re-directed to the new plastids. But the reasons why this happens in dinoflagellates and not in other groups with secondary plastids are entirely obscure.
Classification
Dinoflagellates have been studied and classified by botanists, zoologists and paleontologists, and this has resulted in differing taxonomic practices and dual (or even triple) classification schemes. Fensome et al. (1993) unified dinoflagellate classification.
As in every other group, molecular data have given much insight onto the phylogenetic history of dinoflagellates. However, this has not (yet) been translated into official renaming of higher taxonomic groups. The main reason for this is that most dinoflagellate phylogenetic trees have backbones that are poorly resolved, and so it is difficult to determine phylogenetic relationships of large groups to each other based on this kind of data alone (Daugbjerg et al. 2000, Saldarriaga et al. 2004). In dinoflagellates, the main value of molecular phylogenetic data have been to clarify in-group phylogenies, for example within groups like calciodinellids, pfiesteriaceans, polykrikoids or the genera Symbiodinium or Alexandrium, as well as to underline the differences between groupings of gymnodinoids. In addition, it has been gratifying to see that molecular data generally agree with classifications of gonyaulacalean genera based on tabulation. To a large degree this is also true in peridinioids, but here the situation is complicated by the ‘intromission’ of many prorocentralean, dinophysialean and even gymnodinialean groupings that form a so-called GPP group (gymnodinoids, peridinoids and prorocentroids).
One recent (and very welcome) trend has been the re-investigation of the type species of large, polyphyletic genera of gymnodinoid dinoflagellates like Gymnodinium, Gyrodinium, Amphidinium, with both ultrastructural and molecular methods. This has enabled a more phylogenetically-accurate circumscription of those large genera, and has caused a flood of descriptions of new gymnodinoid genera that are not particularly closely related to those types (e.g. Karenia, Karlodinium, Takayama, Togula, Prosoaulax, Apicoporus, Tovellia, Borghiella, Baldinia, Jadwigia). It should be noted, however, that Gymnodinium, Gyrodinium, Amphidinium, are formally still polyphyletic, they contain many species that have not been re-investigated recently or that have not yet been given new taxonomic placements. Recent papers have used the terms sensu lato and sensu stricto to distinguish between the polyphyletic and the newly-defined versions of these genera. In the case of Gymnodinium, even the ‘sensu stricto’ version of the genus is still paraphyletic, it has been shown that Polykrikos, Pheopolykrikos, Warnowia and Nematodinium are all decended from it (Hoppenrath and Leander 2007, and unpublished data). In this work, as in much of the primary literature, when there is reason to believe that a species is misclassified into a certain genus, that generic name is given inside apostrophes (e.g. ‘Gyrodinium’ dorsum, ‘Amphidinium’ longum, ‘Pheopolykrikos’ hartmanii).
Literature
English-language taxonomic monographs covering large numbers of species are those by Steidinger and Williams (1970, Gulf of Mexico), Taylor (1976, Indian Ocean), Dodge (1982, British Isles), and Gómez (2003, Mediterranean). The taxonomy of extant and fossil species was unified for the first time by Fensome et al. (1993). A good summary of the biology of the group is presented in Hackett et al. (2004b). Papers concerned primarily with the evolution of the whole group include Taylor (2004), Saldarriaga et al. (2004) and Zhang et al. (2005). Two volumes edited by Spector (1984) and Taylor (1987) have brought together much general literature. Major reviews have been provided on particular aspects (e.g. Fensome et al. 1993: classification; Graneli and Turner 2006: biology of harmful species; Coffroth and Santos 2005: zooxanthellae).
References
Akimoto, H., C. Wu, T. Kinumi, and Y. Ohmiya. 2004. Biological rhythmicity in expressed proteins of the marine dinoflagellate L. polyedrum demonstrated by chronological proteomics. Biochem. Biophys. Res. Commun. 315:306-312.
Bachvaroff, T. R., G. T. Concepci�n, C. R. Rogers, E. M. Herman, and C. F. Delwiche. 2004. Dinoflagellate expressed sequence tag data indicate massive transfer of chloroplast genes to the nuclear genome. Protist 155:65-78.
Cavalier-Smith, T. 1991. Cell diversification in heterotrophic flagellates. In Patterson, D. J., and J. Larsen, eds. The biology of free-living heterotrophic flagellates. Syst. Assoc. Special Vol. 45, Clarendon Press, Oxford.
Cavalier-Smith, T. 1999. Principles of protein and lipid targeting in secondary symbiogenesis: euglenoid, dinoflagellate, and sporozoan plastid origins and the eukaryote family tree. J. Eukaryot. Microbiol. 46:347-366.
Cavalier-Smith, T. 2003. Genomic reduction and evolution of novel genetic membranes and protein-targeting machinery in eukaryote-eukaryote chimaeras (meta-algae). Phil. Trans. Roy. Soc. London, Series B 358 (1429):109-133.
Cavalier-Smith, T., and E. E. Chao. 2004. Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates. Eur. J. Protistol. 40:185-212.
Cembella, A. D. 2003. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 42:420-447.
Coffroth, M. A., and S. R. Santos. 2005. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist 156:19-34.
Daugbjerg, N., G. Hansen, J. Larsen, and �. Moestrup. 2000. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia 39:302-317.
Dodge, J. D. 1966. The Dinophyceae. In Godward, M. B. E., ed. The chromosomes of the algae. St. Martin�s Press, New York, USA, pp. 96-115.
Dodge, J. D. 1971. A dinoflagellate with both a mesokaryotic and a eukaryotic nucleus. I. Fine structure of the nuclei. Protoplasma 73:145-157.
Dodge, J. D. 1972. The ultrastructure of the dinoflagellate pusule: a unique osmo-regulatory organelle. Protoplasma 75:285-302.
Dodge, J. D. 1973. The Fine Structure of Algal Cells. London, Academic Press.
Dodge, J. D. 1982. Marine Dinoflagellates of the British Isles. Her Majesty�s Stationary Office, London.
Dodge, J. D. 1987. Dinoflagellate ultrastructure. In Taylor, F. J. R., ed. The biology of dinoflagellates. Botanical Monographs Volume 21, Blackwell Scientific Publications, Oxford.
Dodge, J. D., and R. M. Crawford. 1971. A fine-structural survey of dinoflagellate pyrenoids and food-reserves. Bot. J. Linn. Soc. 64:105-115.
Elbr�chter, M., and E. Schnepf. 1996. Gymnodinium chlorophorum, a new green, bloom-forming dinoflagellate (Gymnodiniales, Dinophyceae) with a vestigial prasinophyte endosymbiont. Phycologia 35:381-393.
Evitt, W. R. 1985. Sporopollenin Dinoflagellate Cysts: Their Morphology and Interpretation. American Association Stratigraphic Palynologists Monograph Ser. 1.
Fast, N. M., J. C. Kissinger, D. S. Roos, and P. J. Keeling. 2001. Nuclear-encoded, plastid-targeted genes suggest a single common origin for apicomplexan and dinoflagellate plastids. Mol. Biol. Evol. 18:418-426.
Fast, N. M., L. Xue, S. Bingham, and P. J. Keeling. 2002. Re-examining alveolate evolution using multiple protein molecular phylogenies. J. Eukaryot. Microbiol. 49:30-37.
Fensome R. A., F. J. R. Taylor, G. Norris, W. A. S. Sarjeant, D. I. Wharton, and G. L. Williams. 1993. A classification of living and fossil dinoflagellates. Micropaleontology Special Publication 7, Sheridan Press, Hanover, Pennsylvania, USA.
Figueroa, R. I., I. Bravo, and E. Garc�s. 2006. Multiple routes of sexuality in Alexandrium taylori (Dinophyceae) in culture. J. Phycol. 42:1028-1039.
Figueroa, R. I., E. Garc�s, and I. Bravo. 2007. Comparative study of the life cycles of Alexandrium tamutum and Alexandrium minutum (Gonyaulacales, Dinophyceae) in culture. J. Phycol. 43:1039-1053.
Francis, D. 1967. On the eyespot of the dinoflagellate, Nematodinium. J. Exp. Biol. 47:495�502.
Gaines, G., and M. Elbr�chter. 1987. Heterotrophic nutrition. In Taylor, F. J. R., ed. The biology of dinoflagellates. Botanical Monographs Volume 21, Blackwell Scientific Publications, Oxford.
Gaines, G., and F. J. R. Taylor. 1984. Extracellular digestion in marine dinoflagellates. J. Plankton Res. 6:1057�1061.
Gaines, G., F. J. R. Taylor. 1985. Form and function of the dinoflagellate transverse flagellum. J. Protozool. 32:290�296.
G�mez, F. 2003. Checklist of Mediterranean free-living dinoflagellates. Bot. Mar. 46:215-242.
Gran�li, E., and J. T. Turner. eds. 2006. Ecology of harmful algae. Ecological Studies, Vol. 189. Berlin Heidelberg, Springer Verlag.
Green, B. R. 2004. The chloroplast genome of dinoflagellates: a reduced instruction set? Protist 155:23-31.
Greuet, C. 1978. Ultrastructural organization of the ocelloid of Nematodinium. Phylogenetic aspect of the evolution of Warnowiidae Lindemann dinoflagellates photoreceptor. Cytobiology 17:114�136.
Greuet, C. 1987. Complex Organelles. In Taylor, F. J. R., ed. The biology of dinoflagellates. Botanical Monographs Volume 21, Blackwell Scientific Publications, Oxford.
Hackett, J. D., H. S. Yoon, M. B. Soares, M. F. Bonaldo, T. Casavant, T. E. Scheetz, T. Nosenko, and D. Bhattacharya. 2004a. Migration of the plastid genome to the nucleus in a peridinin dinoflagellate. Curr. Biol. 14:213-218.
Hackett, J. D., D. M. Anderson, D. L. Erdner, and D. Bhattacharya. 2004b. Dinoflagellates: a remarkable evolutionary experiment. Am. J. Bot. 91:1523-1534.
Hansen, G., L. Botes, and M. de Salas. 2007a. Ultrastructure and large subunit rDNA sequences of Lepidodinium viride reveal a close relationship to Lepidodinium chlorophorum comb. nov. (= Gymnodinium chlorophorum). Phycol. Res. 55:25-41.
Hansen, G., N. Daugbjerg, and P. Henriksen. 2007b. Baldinia anauniensis gen. et sp. nov.: a �new� dinoflagellate from Lake Tovel, N. Italy. Phycologia 46:86-108.
Harper, J. T., and P. J. Keeling. 2003. Nucleus-encoded, plastid-targeted glyceraldehyde-3-phosphate dehydrogenase (GAPDH) indicates a single origin for chromist and alveolate plastids. Mol. Biol. Evol. 20:1730-1735.
Hoppenrath, M., and B. S. Leander. 2007a. Character evolution in polykrikoid dinoflagellates. J. Phycol. 43:366-377.
Hoppenrath, M., and B. S. Leander. 2007b. Morphology and phylogeny of the pseudocolonial dinoflagellates Polykrikos lebourae and Polykrikos herdmanae n. sp. Protist 158:209-227.
Hoppenrath, M., and B. S. Leander. 2008. Morphology and molecular phylogeny of a new marine sand-dwelling Prorocentrum species, P. tsawwassenense (Dinophyceae, Prorocentrales), from British Columbia, Canada. - Journal of Phycology 44: 451-466.
Horiguchi, T., and R. N. Pienaar. 1991. Ultrastructure of a marine dinoflagellate Peridinium quinquecorne Ab� (Peridiniales) from South Africa with particular reference to its crysophyte endosymbiont. Bot. Mar. 34:123-131.
Horiguchi, T., and R. N. Pienaar. 1994. Ultrastructure of a new marine sand-dwelling dinoflagellate, Gymnodinium quadrilobatum sp. nov. (Dinophyceae) with special reference to its endosymbiotic alga. Europ. J. Phycol. 29:237-245.
Howe, C. J., R. E. R. Nisbet, and A. C. Barbrook. 2008. The remarkable chloroplast genome of dinoflagellates. J. Exp. Bot. 59:1035-1045.
Ishida, K., and B. R. Green. 2002. Second- and third-hand chloroplasts in dinoflagellates: phylogeny of oxygen-evolving enhancer 1 (PsbO) protein reveals replacement of a nuclear-encoded plastid gene by that of a haptophyte tertiary endosymbiont. Proc. Nat. Acad. Sci. USA 99:9294-9299.
Jacobson, D. M., and D. M. Anderson. 1992. Ultrastructure of the feeding apparatus and myonemal system of the heterotrophic dinoflagellate Protoperidinium spinulosum. J. Phycol. 28:69-82.
Janson, S. 2004. Molecular evidence that plastids in the toxin-producing dinoflagellate genus Dinophysis originate from the free-living cryptophyte Teleaulax amphioxeia. Environm. Microbiol. 6:1102-1106.
Jeffrey, S. W., M. Sielicki, and F. T. Haxo. 1975. Chloroplast pigment patterns in dinoflagellates. J. Phycol. 11:374-384.
Johnson, C. H., and J. W. Hastings. 1986. The elusive mechanism of the circadian clock. Am. Scient. 74:29-36.
Keeling, P. 2008. Bridge over troublesome plastids. Nature 451, 896-897.
Knaust, R., T. Urbig, L. Li, W. Taylor, and J. W. Hastings. 1998. The circadian rhythm of bioluminescence in Pyrocystis is not due to differences in the amount of luciferase: a comparative study of three bioluminescent marine dinoflagellates. J. Phycol. 34:167-172.
Koike, K., H. Sekiguchi, A. Kobiyama, K. Takishita, M. Kawachi, K. Koike, and T. Ogata. 2005. A novel type of kleptoplastidy in Dinophysis (Dinophyceae): presence of a haptophyte-type plastid in Dinophysis mitra. Protist 156:225-237.
Kuvardina, O. N., B. S. Leander, V. V. Aleshin, A. P. Mylnikov, P. J. Keeling, and T. G. Simdyanov. 2002. The phylogeny of colpodellids (Alveolata) using small-subunit rRNA gene sequences suggests they are the free-living sister group to apicomplexans. J. Eukaryot. Microbiol. 49:498-504.
Leander, B. S., and P. J. Keeling. 2003. Morphostasis in alveolate evolution. Trends Ecol. Evol. 18:395-402.
Leblond, P. H., and F. J. R. Taylor. 1976. The propulsive mechanism of the dinoflagellate transverse flagellum reconsidered. BioSystems 8:33-39.
Lehane, L., and R. J. Lewis. 2000. Ciguatera: recent advances, but the risk remains. Int. J. Food Microbiol. 61:91-125.
Lin, S., H. Zhang, D. F. Spencer, J. E. Norman, M. W. Grey. 2002. Widespread and extensive editing of mitochondrial mRNAs in dinoflagellates. J. Mol. Biol. 320:727-739.
Livolant, F. 1982a. Dinoflagellate trichocyst ultrastructure I. The shaft. Biol. Cell 43:201�210.
Livolant, F. 1982b. Dinoflagellate trichocyst ultrastructure II. Existence of a sheath. Biol. Cell 43:211�216.
Logares, R., K. Schalchian-Tabrizi, A. Boltovskoy, and K. Rengefors. 2007. Extensive dinoflagellate phylogenies indicate infrequent marine-freshwater transitions. Mol. Phyl. Evol. 45:887-903.
Matsuzaki, M., H. Kuroiwa, T. Kuroiwa, K. Kita, and H. Nozaki. 2008. A cryptic algal group unveiled: a plastid biosynthesis pathway in the oyster parasite Perkinsus marinus. Mol. Biol. Evol. Advanced Access.
Moestrup, �., G. Hansen, and N. Daugbjerg. 2008. Studies on woloszynskioid dinoflagellates. III: On the ultrastructure and phylogeny of Borghiella dodgei gen. et sp. nov., a cold-water species from Lake Tovel, N. Italy, and on B. tenuissima comb. nov. (syn. Woloszynskia tenuissima). Phycologia 47:54-78.
Moore, R. B., M. Oborn�k, J. Janu�kovec, T. Chrudimsk�, M. Vancov�, D. H. Green, S. W. Wright, N. W. Davies, C. J. S. Bolch, K. Heimann, J. �lapeta, O. Hoegh-Guldberg, Jr. J. M. Logsdon, and D. A. Carter. 2008. A photosynthetic alveolate closely related to apicomplexan parasites. Nature 451:959-963.
Moreira, D., and P. L�pez-Garc�a. 2002. The molecular ecology of microbial eukaryotes unveils a hidden world. Trends Microbiol. 10:31-38.
Morrill, L. C., and A. R. Loeblich III. 1983. Ultrastructure of the dinoflagellate amphiesma. Int. Rev. Cytol. 82:151�181.
Morse, D., P. Salois, P. Markovic, and J. W. Hastings. 1995. A nuclear-encoded form II rubisco in dinoflagellates. Science 268:1622-1624.
Nash, E. A., A. C.Barbrook, R. K. Edwards-Stewart, K. Bernhardt, C. J. Howe, and R. E. Nisbet. 2007. Organization of the mitochondrial genome in the dinoflagellate Amphidinium carterae. Mol. Biol. Evol. 24:1528-1536.
Netzel, H., and G. D�rr. 1984. Dinoflagellate cell cortex. In Spector, D. L., ed. Dinoflagellates. Chapt. 3. New York: Academic Press.
Nisbet, R. E. R., V. L. Koumadou, A. C. Barbrook, and C. J. Howe. 2004. Novel plastid gene minicircles in the dinoflagellate Amphidinium operculatum. Gene 331:141-147.
Nosenko, T., K. L. Lidie, F. M. Van Dolah, E. Lindquist, J. F. Cheng, and D. Bhattacharya. 2006. Chimeric plastid proteome in the Florida red tide dinoflagellate Karenia brevis. Mol. Biol. Evol. 23:2026-2038.
Patron, N. J., R. F. Waller, and P. J. Keeling. 2006. A tertiary plastid uses genes from two endosymbionts. J. Mol. Biol. 357:1373-1382.
Poupin, J., A.-S. Cussatlegras, and P. Geistdoerfer. 1999. Plancton marin bioluminescent. Rapport scientifique du Laboratoire d�Oc�anographie de l��cole Navale LOEN, Brest, France, 83 pp.
Rizzo, P. J. 1991. The enigma of the dinoflagellate chromosome. J. Protozool. 38:246-252.
Saldarriaga, J. F., F. J. R. Taylor, P. J. Keeling, and T. Cavalier-Smith. 2001. Dinoflagellate nuclear SSU rRNA phylogeny suggests multiple chloroplast losses and replacements. J. Mol. Evol. 53:204-213.
Saldarriaga, J. F., M. L. McEwan, N. M. Fast, F. J. R. Taylor, and P. J. Keeling. 2003. Multiple protein phylogenies show that Oxyrrhis marina and Perkinsus marinus are early branches of the dinoflagellate lineage. Int. J. Syst. Evol. Microbiol. 53:355-365.
Saldarriaga, J. F., F. J. R. Taylor, T. Cavalier-Smith, S. Menden-Deuer, and P. J. Keeling. 2004. Molecular data and the evolutionary history of dinoflagellates. Eur. J. Protistol. 40:85-111.
S�nchez-Puerta, M. V., J. C. Lippmeier, K. E. Apt, and C. F. Delwiche. 2007. Plastid genes in a non-photosynthetic dinoflagellate. Protist 158:105-117.
Schnepf, E., and M. Elbr�chter. 1988. Cryptophycean-like double-membrane-bound chloroplast in the dinoflagellate, Dinophysis Ehrenb.: evolutionary, phylogenetic and toxicological implications. Bot. Acta 101:196-203.
Schnepf, E., and M. Elbr�chter. 1992. Nutritional strategies in dinoflagellates: a review with emphasis on cell biological aspects. Eur. J. Protistol. 28:3-24.
Schnepf, E., and M. Elbr�chter. 1999. Dinophyte chloroplasts and phylogeny-a review. Grana 38:81-97.
Schweikert, M., and M. Elbr�chter. 2004. First ultrastructural investigations of the consortium between a phototrophic eukaryotic endosymbiont and Podolampas bipes (Dinophyceae). Phycologia 43:614-623.
Slamovits, C. H., J. F. Saldarriaga, A. Larocque, and P. J. Keeling. 2007. The highly reduced and fragmented mitochondrial genome of the early-branching dinoflagellate Oxyrrhis marina shares characteristics with both apicomplexan and dinoflagellate mitochondrial genomes. J. Mol. Biol. 372:356-368.
Slamovits, C. H., and P. J. Keeling. 2008. Plastid-derived genes in the non-photosynthetic alveolate Oxyrrhis marina. Mol. Biol. Evol. in press.
Smayda, T.J. 1997. What is a bloom? A commentary. Limnol. Oceanogr. 42:1132-1136.
Sparmann, S. F., B. S. Leander, and M. Hoppenrath. 2008. Comparative morphology and molecular phylogeny of Apicoporus n. gen.: a new genus of marine benthic dinoflagellates formerly classified within Amphidinium. Protist 159:383-399.
Spector, D. L., ed. 1984. Dinoflagellates. New York, Academic Press.
Spector, D. L., A. C. Vasconcelos, and R. E. Triemer. 1981. DNA duplication and chromosome structure in the dinoflagellates. Protoplasma 105:185�194.
Steidinger, K. A., and J. Williams. 1970. Memoirs of the Hourglass Cruises. Vol. II, Marine Res. Lab., Florida.
Stelter, K., N. M. El-Sayed, and F. Seeber. 2007. The expression of a plant-type ferredoxin redox system provides molecular evidence for a plastid in the early dinoflagellate Perkinsus marinus. Protist 158:119-130.
Sweeney, B. M. 1987. Bioluminescence and circadian rhythms. In Taylor, F. J. R., ed. The Biology of Dinoflagellates. Botanical Monographs, Oxford: Blackwell Scientific Publ.
Taylor, F. J. R. 1975. Non-helical transverse flagella in dinoflagellates. Phycologia 14:45-47.
Taylor, F. J. R. 1976. Dinoflagellates from the International Indian Ocean Expedition. Biblioteca Botanica 132:1-234, pls. 1-46.
Taylor, F. J. R. 1980. On dinoflagellate evolution. BioSystems 13:65-108.
Taylor, F. J. R., ed. 1987. The Biology of Dinoflagellates. Botanical Monographs, Oxford, Blackwell Scientific Publ.
Taylor, F. J. R. 2004. Illumination or confusion? Dinoflagellate molecular phylogenetic data viewed from a primarily morphological standpoint. Phycol. Res. 52:308-324.
Taylor, F. J. R., M. Hoppenrath, and J. F. Saldarriaga. 2008. Dinoflagellate diversity and distribution. Biodiv. Cons. 17:407-418.
Tengs, T., O. J. Dahlberg, K. Shalchian-Tabrizi, D. Klaveness, K. Rudi, C. F. Delwiche, and K. S. Jakobsen. 2000. Phylogenetic analyses indicate that the 19� hexanoyloxyfucoxanthin-containing dinoflagellates have tertiary plastids of haptophyte origin. Mol. Biol. Evol. 17:718-729.
Trench, R. K. 1997. Diversity of symbiotic dinoflagellates and the evolution of microalgal-invertebrate symbioses. In Lessios, H. A., and I. G. MacIntyre eds. Proceedings of the eighth international coral reef symposium 2:1275-1286. Smithsonian Tropical Research Institute, Balboa, Panama.
Watanabe, M. M., T. Sasa, S. Suda, I. Inouye, and S. Takishi. 1991. Major carotenoid composition of an endosymbiont in a green dinoflagellate, Lepidodinium viride. J. Phycol. 27:75.
Westfall, J. A., P. C. Bradbury, and J. W. Townsend. 1983. Ultrastructure of the dinoflagellate Polykrikos. J. Cell Sci. 63:245�261.
Whitney, S. M., D. C. Shaw, and D. Yellowlees. 1995. Evidence that some dinoflagellates contain a ribulose-1,5-biphosphate carboxylase/oxygenase related to that of the alpha-proteobacteria. Proc. Royal Soc. London, Ser. B 259:271-275.
Worden, A. Z. 2006. Picoeukaryote diversity in coastal waters of the Pacific Ocean. Aquatic Microb. Ecol. 43:165-175.
Zhang, H., D. Bhattacharya, and S. Lin. 2005. Phylogeny of dinoflagellates based on mitochondrial cytochrome b and nuclear small subunit rDNA sequence comparisons. J. Phycol. 41:411-420.
Zhang, H., and S. Lin. 2005. Mitochondrial cytochrome b mRNA editing in dinoflagellates: possible ecological and evolutionary associations? J. Eukaryot. Microbiol. 52:538-545.
Zhang, Z., B. R. Green, and T. Cavalier-Smith. 1999. Single gene circles in dinoflagellate plastid genomes. Nature 400:155-159.
Information on the Internet
- Centre of Excellence for Dinophyte Taxonomy CEDiT. Forschungsinstitut Senckenberg, German Centre for Marine Biodiversity Research
- Plankton*net@AWI. Alfred Wegener Institute for Polar and Marine Research
- International Society for the Study of Harmful Algae
- Harmful Algae. Woods Hole Oceanographic Institute.
- Harmful Algal Bloom Programme. Intergovernmental Algal Bloom Programme.
- Dinoflagellate Photographs, Atlas, and Technical Guides
- The World of Dinoflagellates
- The Freshwater Dinoflagellates
- Dinoflagellates. Andrew MacRae, University of Calgary.
- Growing dinoflagellates at home. The Bioluminescence Web Page.
- Dinoflagellates collections at Micro*scope
Title Illustrations

| Scientific Name | Togula britannica |
|---|---|
| Specimen Condition | Live Specimen |
| Identified By | Mona Hoppenrath |
| Copyright |
© Mona Hoppenrath

|
| Scientific Name | Ceratium tripos |
|---|---|
| Specimen Condition | Live Specimen |
| Identified By | Mona Hoppenrath |
| Copyright |
© Mona Hoppenrath

|
| Scientific Name | 'Gymnodinium' sp. |
|---|---|
| Specimen Condition | Live Specimen |
| Identified By | Mona Hoppenrath |
| Copyright |
© Mona Hoppenrath

|
| Scientific Name | Dinophysis tripos |
|---|---|
| Specimen Condition | Live Specimen |
| Identified By | Mona Hoppenrath |
| Copyright |
© Mona Hoppenrath

|
| Scientific Name | Prorocentrum lima |
|---|---|
| Copyright |
© Mona Hoppenrath

|
About This Page
This page is being developed as part of the Tree of Life Web Project Protist Diversity Workshop, co-sponsored by the Canadian Institute for Advanced Research (CIFAR) program in Integrated Microbial Biodiversity and the Tula Foundation.
Mona Hoppenrath

Forschungsinstitut Senckenberg, German Centre for Marine Biodiversity Research, Wilhelmshaven, Germany

University of British Columbia, Vancouver, British Columbia, Canada
Correspondence regarding this page should be directed to Mona Hoppenrath at and Juan F. Saldarriaga at
Page copyright © 2012 Mona Hoppenrath and
 Page: Tree of Life
Dinoflagellates.
Authored by
Mona Hoppenrath and Juan F. Saldarriaga.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Dinoflagellates.
Authored by
Mona Hoppenrath and Juan F. Saldarriaga.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 05 August 2008
- Content changed 15 December 2012
Citing this page:
Hoppenrath, Mona and Juan F. Saldarriaga. 2012. Dinoflagellates. Version 15 December 2012 (under construction). http://tolweb.org/Dinoflagellates/2445/2012.12.15 in The Tree of Life Web Project, http://tolweb.org/




























 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site