Thietane
Appearance
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Thietane | |||
| Systematic IUPAC name
Thiacyclobutane | |||
| Other names
Trimethylene sulfide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 102383 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.469 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 1993 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6S | |||
| Molar mass | 74.14 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Sulfurous | ||
| Density | 1.028 g cm−3 | ||
| Boiling point | 94 to 95 °C (201 to 203 °F; 367 to 368 K) | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H225, H302 | |||
| P210 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | -11(9) °C | ||
| Related compounds | |||
Other anions
|
Oxetane, Azetidine, Phosphetane | ||
Related compounds
|
Thiirane, Dithietane, Tetrahydrothiophene, Thiane, Thiepane, Thiocane, Thionane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
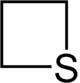
Thietane is a heterocyclic compound containing a saturated four-membered ring with three carbon atoms and one sulfur atom.[1][2]
Thietane, and its derivative 2-propylthietane, are strong-smelling mouse alarm pheromones and predator scent analogues.[3][4] Both the mouse and human olfactory receptors MOR244-3 and OR2T11, respectively, were found to respond to thietane in the presence of copper.[5]
Synthesis
[edit]Thietane can be produced from the reaction of trimethylene carbonate and potassium thiocyanate, but the yield is low.[6]
An improved synthesis method is the reaction of 1,3-dibromopropane and sodium sulfide.[7]
Reactions
[edit]Nucleophiles like butyllithium can open the ring in thietane.[8] Thietane also reacts with bromine.[9]
References
[edit]- ^ Leśniak, S; Lewkowski, J; Kudelska, W; Zając, A (2008). "Thietanes and Thietes: Monocyclic". Comprehensive Heterocyclic Chemistry III. 2 (7): 389–428. doi:10.1016/B978-008044992-0.00207-8.
- ^ Block, E; DeWang, M (1996). "Thietanes and Thietes: Monocyclic". Comprehensive Heterocyclic Chemistry II. 1 (24): 773–802. doi:10.1016/B978-008096518-5.00024-1.
- ^ Sievert, Thorbjörn; Laska, Matthias (2016). "Behavioral responses of CD-1 mice to six predator odor components". Chem. Senses. 41 (5): 399–406. doi:10.1093/chemse/bjw015. PMID 26892309.
- ^ Brechbuhl, J; Moine, F; Klaey, M; Nenniger-Tosato, M; Hurni, N; Sporkert, F; Giroud, C; Broillet, MC (2013). "Mouse alarm pheromone shares structural similarity with predator scents". Proc. Natl. Acad. Sci. U.S.A. 110 (12): 4762–4767. Bibcode:2013PNAS..110.4762B. doi:10.1073/pnas.1214249110. PMC 3607058. PMID 23487748.
- ^ Li, Shengju; Ahmed, Lucky; Zhang, Ruina; Pan, Yi; Matsunami, Hiroaki; Burger, Jessica L; Block, Eric; Batista, Victor S; Zhuang, Hanyi (2016). "Smelling sulfur: Copper and silver regulate the response of human odorant receptor OR2T11 to low molecular weight thiols". Journal of the American Chemical Society. 138 (40): 13281–13288. doi:10.1021/jacs.6b06983. PMID 27659093.
- ^ Searles, Scott; Lutz, Eugene F. (1958). "A New Synthesis of Small Ring Cyclic Sulfides". Journal of the American Chemical Society. 80 (12): 3168. doi:10.1021/ja01545a071. ISSN 0002-7863.
- ^ Nagasawa, Kazuo; Yoneta, Akemi (1985). "Organosulfur chemistry. II. Use of dimethyl sulfoxide; A facile synthesis of cyclic sulfides". Chemical and Pharmaceutical Bulletin. 33 (11): 5048–5052. doi:10.1248/cpb.33.5048. ISSN 0009-2363.
- ^ Bordwell, F. G.; Andersen, Harry M.; Pitt, Burnett M. (1954). "The Reaction of Thiacyclopropanes (Olefin Sulfides) and Thiacyclobutanes with Organolithium Compounds". Journal of the American Chemical Society. 76 (4): 1082–1085. doi:10.1021/ja01633a045. ISSN 0002-7863.
- ^ Stewart, John M.; Burnside, Charles H. (1953). "Reactions of Trimethylene Sulfide with Chlorine and Bromine". Journal of the American Chemical Society. 75 (1): 243–244. doi:10.1021/ja01097a517. ISSN 0002-7863.