In organic chemistry, the thiol-yne reaction (also known as alkyne hydrothiolation) is an organic reaction between a thiol (−SH) and an alkyne (−C≡CH). The reaction product is an alkenyl sulfide (−CH=CH−S−).[1][2]
The reaction was first reported in 1949 with thioacetic acid as reagent[3][4] and rediscovered in 2009.[5] It is used in click chemistry[6][7][8] and in polymerization, especially with dendrimers.
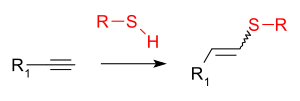 |
| Thiol-yne reaction |
|---|
This addition reaction is typically facilitated by a radical initiator or UV irradiation and proceeds through a sulfanyl radical species. With monoaddition a mixture of (E/Z)-alkenes form. The mode of addition is anti-Markovnikov. The radical intermediate can engage in secondary reactions such as cyclisation.[9][10] With diaddition the 1,2-disulfide or the 1,1- dithioacetal forms. Reported catalysts for radical additions are triethylborane,[11] indium(III) bromide[12] and AIBN.[13] The reaction is also reported to be catalysed by cationic rhodium and iridium complexes,[14] by thorium and uranium complexes,[15] by rhodium complexes,[16][17][18] by caesium carbonate[19] and by gold.[20]
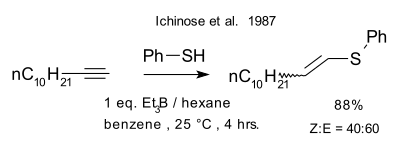 |
| Ichinose et al. thiol-yne reaction 1987[11] |
|---|
Diphenyl disulfide reacts with alkynes to a 1,2-bis(phenylthio)ethylene.[21] Reported alkynes are ynamides.[22] A photoredox thiol-yne reaction has been reported.[23]
Polymer chemistry
editIn polymer chemistry, systems have been described based on addition polymerization with 1,4-benzenedithiol and 1,4-diethynylbenzene,[24][25] in the synthesis of other addition polymer systems[26] in the synthesis of dendrimers,[27][28][29][30] in star polymers,[31][32][33][34] in graft polymerization,[35] block copolymers,[36] and in polymer networks.[5][37] Another reported application is the synthesis of macrocycles via dithiol coupling.[38]
See also
editReferences
edit- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595
- ^ Hoogenboom, Richard (2010). "Thiol-Yne Chemistry: A Powerful Tool for Creating Highly Functional Materials". Angewandte Chemie International Edition. 49 (20): 3415–7. doi:10.1002/anie.201000401. PMID 20394091.
- ^ Bader, H.; Cross, L. C.; Heilbron, Ian; Jones, E. R. H. (1949). "132. Researches on acetylenic compounds. Part XVIII. The addition of thiolacetic acid to acetylenic hydrocarbons. The conversion of monosubstituted acetylenes into aldehydes and 1 : 2-dithiols". Journal of the Chemical Society (Resumed): 619. doi:10.1039/JR9490000619.
- ^ Bader, Henry (1956). "23. The addition of thiolacetic acid to ethynylcarbinols and the conversion of the adducts into aldols and ??-unsaturated aldehydes". Journal of the Chemical Society (Resumed): 116–121. doi:10.1039/JR9560000116.
- ^ a b Fairbanks, Benjamin D.; Scott, Timothy F.; Kloxin, Christopher J.; Anseth, Kristi S.; Bowman, Christopher N. (2009). "Thiol−Yne Photopolymerizations: Novel Mechanism, Kinetics, and Step-Growth Formation of Highly Cross-Linked Networks". Macromolecules. 42 (1): 211–217. Bibcode:2009MaMol..42..211F. doi:10.1021/ma801903w. PMC 2651690. PMID 19461871.
- ^ Lowe, Andrew B.; Hoyle, Charles E.; Bowman, Christopher N. (2010). "Thiol-yne click chemistry: A powerful and versatile methodology for materials synthesis". Journal of Materials Chemistry. 20 (23): 4745. doi:10.1039/B917102A.
- ^ Lowe, Andrew B. (October 2014). "Thiol-yne 'click'/coupling chemistry and recent applications in polymer and materials synthesis and modification". Polymer. 55 (22): 5517–5549. doi:10.1016/j.polymer.2014.08.015.
- ^ Yao, BiCheng; Sun, JingZhi; Qin, AnJun; Tang, Ben Zhong (11 June 2013). "Thiol-yne click polymerization". Chinese Science Bulletin. 58 (22): 2711–2718. Bibcode:2013ChSBu..58.2711Y. doi:10.1007/s11434-013-5892-1.
- ^ Montevecchi, P; Navacchia, M (1998). "Sulfanyl radical mediated cyclization of aminyl radicals". Tetrahedron Letters. 39 (49): 9077. doi:10.1016/S0040-4039(98)01998-4.
- ^ Taniguchi, Tsuyoshi; Fujii, Tatsuya; Idota, Atsushi; Ishibashi, Hiroyuki (2009). "Reductive Addition of the Benzenethiyl Radical to Alkynes by Amine-Mediated Single Electron Transfer Reaction to Diphenyl Disulfide". Organic Letters. 11 (15): 3298–301. doi:10.1021/ol901084k. PMID 19719181.
- ^ a b Ichinose, Yoshifumi; Wakamatsu, Kuni; Nozaki, Kyoko; Birbaum, Jean-Luc; Oshima, Koichiro; Utimoto, Kiitiro (1987). "Et3B induced radical addition of thiols to acetylenes". Chemistry Letters. 16 (8): 1647–1650. doi:10.1246/cl.1987.1647.
- ^ Yadav, J. S.; Reddy, B. V. Subba; Raju, A.; Ravindar, K.; Baishya, Gakul (2007). "Hydrothiolation of Unactivated Alkynes Catalyzed by Indium(III) Bromide". Chemistry Letters. 36 (12): 1474. doi:10.1246/cl.2007.1474.
- ^ Benati, Luisa; Capella, Laura; Montevecchi, Pier Carlo; Spagnolo, Piero (1995). "Free-Radical Addition of Heteroarenethiols and Heteroarylmethanethiols to Hexyne and Phenylacetylene. Chemical Behavior of the Transient .beta.-Sulfanylvinyl Radicals". The Journal of Organic Chemistry. 60 (24): 7941. doi:10.1021/jo00129a039.
- ^ Field, Leslie D.; Messerle, Barbara A.; Vuong, Khuong Q.; Turner, Peter (2009). "Rhodium(I) and iridium(I) complexes containing bidentate phosphine-imidazolyl donor ligands as catalysts for the hydroamination and hydrothiolation of alkynes". Dalton Transactions (18): 3599–614. doi:10.1039/b821188d. PMID 19381423.
- ^ Weiss, Charles J.; Wobser, Stephen D.; Marks, Tobin J. (2009). "Organoactinide-Mediated Hydrothiolation of Terminal Alkynes with Aliphatic, Aromatic, and Benzylic Thiols". Journal of the American Chemical Society. 131 (6): 2062–3. doi:10.1021/ja808764q. PMID 19170549.
- ^ Yang, Jun; Sabarre, Anthony; Fraser, Lauren R.; Patrick, Brian O.; Love, Jennifer A. (2009). "Synthesis of 1,1-Disubstituted Alkyl Vinyl Sulfides via Rhodium-Catalyzed Alkyne Hydrothiolation: Scope and Limitations". The Journal of Organic Chemistry. 74 (1): 182–7. doi:10.1021/jo801644s. hdl:2429/5534. PMID 19053611.
- ^ Sabarre, Anthony; Love, Jennifer (2008). "Synthesis of 1,1-Disubstituted Olefins via Catalytic Alkyne Hydrothiolation/Kumada Cross-Coupling". Organic Letters. 10 (18): 3941–4. doi:10.1021/ol8012843. PMID 18702501.
- ^ Cao, Changsheng; Fraser, Lauren R.; Love, Jennifer A. (2005). "Rhodium-Catalyzed Alkyne Hydrothiolation with Aromatic and Aliphatic Thiols". Journal of the American Chemical Society. 127 (50): 17614–5. doi:10.1021/ja055096h. PMID 16351085.
- ^ Kondoh, Azusa; Takami, Kazuaki; Yorimitsu, Hideki; Oshima, Koichiro (2005). "Stereoselective Hydrothiolation of Alkynes Catalyzed by Cesium Base: Facile Access to (Z)-1-Alkenyl Sulfides". The Journal of Organic Chemistry. 70 (16): 6468–73. doi:10.1021/jo050931z. PMID 16050711.
- ^ Corma, Avelino; González-Arellano, Camino; Iglesias, Marta; Sánchez, Félix (2010). "Efficient synthesis of vinyl and alkyl sulfides via hydrothiolation of alkynes and electron-deficient olefins using soluble and heterogenized gold complexes catalysts". Applied Catalysis A: General. 375: 49–54. doi:10.1016/j.apcata.2009.12.016.
- ^ Benati, Luisa; Montevecchi, Pier Carlo; Spagnolo, Piero (1991). "Free-radical reactions of benzenethiol and diphenyl disulphide with alkynes. Chemical reactivity of intermediate 2-(phenylthio)vinyl radicals". Journal of the Chemical Society, Perkin Transactions 1 (9): 2103. CiteSeerX 10.1.1.1028.9326. doi:10.1039/P19910002103.
- ^ Sato, Akinori; Yorimitsu, Hideki; Oshima, Koichiro (2010). "Radical Additions of Arenethiols to Ynamides for the Selective Synthesis of N-[(Z)-2-(Arylsulfanyl)-1-alkenyl]amides". Bulletin of the Korean Chemical Society. 31 (3): 570. doi:10.5012/bkcs.2010.31.03.570.
- ^ Zalesskiy, Sergey S.; Shlapakov, Nikita S.; Ananikov, Valentine P. (2016). "Visible light mediated metal-free thiol–yne click reaction". Chem. Sci. 7 (11): 6740–6745. doi:10.1039/C6SC02132H. PMC 5355861. PMID 28451118.
- ^ Ohashi, Toyoshi; Kobayashi, Eiichi; Jinbo, Tomoko; Furukawa, Junji (1997). "The crystal structure of 1,4-benzenedithiol by rietveld analysis and studies on the mechanism of solid-state addition polymerization of 1,4-benzenedithiol to 1,4-diethynylbenzene". Journal of Polymer Science Part A: Polymer Chemistry. 35 (9): 1621. Bibcode:1997JPoSA..35.1621O. doi:10.1002/(SICI)1099-0518(19970715)35:9<1621::AID-POLA3>3.0.CO;2-U.
- ^ Kobayashi, Eiichi; Yoshino, Toshizumi; Aoshima, Sadahito; Furukawa, Junji (1995). "Addition polymerization of 2-cyano-1, 4-benzenedithiol to 1,4-diethynylbenzene and properties of polymers". Journal of Polymer Science Part A: Polymer Chemistry. 33 (14): 2403. Bibcode:1995JPoSA..33.2403K. doi:10.1002/pola.1995.080331413.
- ^ Yao, Bicheng; Mei, Ju; Li, Jie; Wang, Jian; Wu, Haiqiang; Sun, Jing Zhi; Qin, Anjun; Tang, Ben Zhong (25 February 2014). "Catalyst-Free Thiol–Yne Click Polymerization: A Powerful and Facile Tool for Preparation of Functional Poly(vinylene sulfide)s". Macromolecules. 47 (4): 1325–1333. Bibcode:2014MaMol..47.1325Y. doi:10.1021/ma402559a.
- ^ Konkolewicz, Dominik; Gray-Weale, Angus; Perrier, SéBastien (2009). "Hyperbranched Polymers by Thiol−Yne Chemistry: From Small Molecules to Functional Polymers". Journal of the American Chemical Society. 131 (50): 18075–7. doi:10.1021/ja908206a. PMID 19947636.
- ^ Chen, Gaojian; Kumar, Jatin; Gregory, Andrew; Stenzel, Martina H. (2009). "Efficient synthesis of dendrimers via a thiol–yne and esterification process and their potential application in the delivery of platinum anti-cancer drugs". Chemical Communications (41): 6291–3. doi:10.1039/b910340f. PMID 19826698.
- ^ Semsarilar, Mona; Ladmiral, Vincent; Perrier, SéBastien (2010). "Highly Branched and Hyperbranched Glycopolymers via Reversible Addition−Fragmentation Chain Transfer Polymerization and Click Chemistry". Macromolecules. 43 (3): 1438. Bibcode:2010MaMol..43.1438S. doi:10.1021/ma902587r.
- ^ Yu, Bing; Chan, Justin W.; Hoyle, Charles E.; Lowe, Andrew B. (2009). "Sequential thiol-ene/thiol-ene and thiol-ene/thiol-yne reactions as a route to well-defined mono and bis end-functionalized poly(N-isopropylacrylamide)". Journal of Polymer Science Part A: Polymer Chemistry. 47 (14): 3544. Bibcode:2009JPoSA..47.3544Y. doi:10.1002/pola.23436.
- ^ Naik, Sandeep S.; Chan, Justin W.; Comer, Christopher; Hoyle, Charles E.; Savin, Daniel A. (2011). "Thiol–yne 'click' chemistry as a route to functional lipid mimetics". Polym. Chem. 2 (2): 303–305. doi:10.1039/C0PY00231C.
- ^ Konkolewicz, Dominik; Gaillard, Sylvain; West, Andrew G.; Cheng, Yuen Yap; Gray-Weale, Angus; Schmidt, Timothy W.; Nolan, Steven P.; Perrier, Sébastien (28 March 2011). "Luminescent Hyperbranched Polymers: Combining Thiol-Yne Chemistry with Gold-Mediated C−H Bond Activation". Organometallics. 30 (6): 1315–1318. doi:10.1021/om200103f.
- ^ Brummelhuis, Niels ten; Schlaad, Helmut (2011). "Stimuli-responsive star polymers through thiol–yne core functionalization/crosslinking of block copolymer micelles". Polymer Chemistry. 2 (5): 1180. doi:10.1039/C1PY00002K.
- ^ Hartlieb, Matthias; Floyd, Thomas; Cook, Alexander B.; Sanchez-Cano, Carlos; Catrouillet, Sylvain; Burns, James A.; Perrier, Sébastien (2017). "Well-defined hyperstar copolymers based on a thiol–yne hyperbranched core and a poly(2-oxazoline) shell for biomedical applications" (PDF). Polymer Chemistry. 8 (13): 2041–2054. doi:10.1039/C7PY00303J.
- ^ Hensarling, Ryan M.; Doughty, Vanessa A.; Chan, Justin W.; Patton, Derek L. (2009). ""Clicking" Polymer Brushes with Thiol-yne Chemistry: Indoors and Out". Journal of the American Chemical Society. 131 (41): 14673–5. doi:10.1021/ja9071157. PMID 19778016.
- ^ Konkolewicz, Dominik; Poon, Cheuk Ka; Gray-Weale, Angus; Perrier, Sébastien (2011). "Hyperbranched alternating block copolymers using thiol–yne chemistry: materials with tuneable properties". Chem. Commun. 47 (1): 239–241. doi:10.1039/C0CC02429E. PMID 20820536.
- ^ Lang, Mathias; Schade, Alexandra; Bräse, Stefan (2016). "Synthesis of three-dimensional porous hyper-crosslinked polymers via thiol–yne reaction". Beilstein Journal of Organic Chemistry. 12: 2570–2576. doi:10.3762/bjoc.12.252. PMC 5238528. PMID 28144326.
- ^ Zhou, Weidong; Zheng, Haiyan; Li, Yongjun; Liu, Huibiao; Li, Yuliang (17 September 2010). "Synthesis of Sulfuric Macrocycles and a Rotaxane through Thiol-yne Click and Dithiol Coupling Reactions". Organic Letters. 12 (18): 4078–4081. doi:10.1021/ol1014569. PMID 20712302.