This article needs additional citations for verification. (December 2009) |
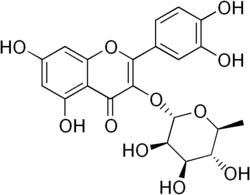
Quercitrin is a glycoside formed from the flavonoid quercetin and the deoxy sugar rhamnose.
| |

| |
| Names | |
|---|---|
| IUPAC name
3′,4′,5,7-Tetrahydroxy-3-(α-L-rhamnopyranosyloxy)flavone
| |
| Systematic IUPAC name
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names
Quercetin 3-O-a-L-rhamnoside
Thujin Quercetin 3-rhamnoside Quercetin-3-rhamnoside Quercetin-3-L-rhamnoside | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.567 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H20O11 | |
| Molar mass | 448.38 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Austrian chemist Heinrich Hlasiwetz (1825-1875) is remembered for his chemical analysis of quercitrin.
It has also been investigated as a potential dietary supplement.[1]
Occurrence
editQuercitrin is a constituent of the dye quercitron. It can be found in Tartary buckwheat (Fagopyrum tataricum)[2] and in oaks species like the North American white oak (Quercus alba) and English oak (Quercus robur).[3] It is also found in Nymphaea odorata or Taxillus kaempferi.[4]
Metabolism
editThe enzyme quercitrinase catalyzes the chemical reaction between quercitrin and H2O to yield L-rhamnose and quercetin.
References
edit- ^ Audah KA, Ettin J, Darmadi J, Azizah NN, Anisa AS, Hermawan TDF; et al. (2022). "Indonesian Mangrove Sonneratia caseolaris Leaves Ethanol Extract Is a Potential Super Antioxidant and Anti Methicillin-Resistant Staphylococcus aureus Drug". Molecules. 27 (23): 8369. doi:10.3390/molecules27238369. PMC 9735687. PMID 36500458.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tartary Buckwheat (Fagopyrum tataricum Gaertn.) as a Source of Dietary Rutin and Quercitrin. Nina Fabjan, Janko Rode, Iztok Jože Košir, Zhuanhua Wang, Zheng Zhang and Ivan Kreft, J. Agric. Food Chem., 2003, 51 (22), pp. 6452–6455, doi:10.1021/jf034543e
- ^ Analysis of oak tannins by liquid chromatography-electrospray ionisation mass spectrometry. Pirjo Mämmelä, Heikki Savolainen, Lasse Lindroos, Juhani Kangas and Terttu Vartiainen, Journal of Chromatography A, Volume 891, Issue 1, 1 September 2000, Pages 75-83, doi:10.1016/S0021-9673(00)00624-5
- ^ The constituents of Taxillus kaempferi and the host, Pinus thunbergii. I. Catechins and flavones of Taxillus kaempferi. Konishi T, Nishio T, Kiyosawa S, Fujiwara Y and Konoshima T, Yakugaku Zasshi., February 1996, volume 116, issue 2, pages 148-157 (article in Japanese), doi:10.1248/yakushi1947.116.2_148