Cystathionine is an intermediate in the synthesis of cysteine from homocysteine. It is produced by the transsulfuration pathway and is converted into cysteine by cystathionine gamma-lyase (CTH).
| |

| |
| Names | |
|---|---|
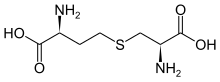
| IUPAC name
S-((R)-2-amino-2-carboxyethyl)-L-homocysteine
| |
| Other names
L-Cystathionine; S-[(2R)-2-Amino-2-carboxyethyl]-L-homocysteine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.269 |
| KEGG | |
| MeSH | Cystathionine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H14N2O4S | |
| Molar mass | 222.26 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Biosynthetically, cystathionine is generated from homocysteine and serine by cystathionine beta synthase (upper reaction in the diagram below). It is then cleaved into cysteine and α-ketobutyrate by cystathionine gamma-lyase (lower reaction).
An excess of cystathionine in the urine is called cystathioninuria.

Cysteine dioxygenase (CDO), and sulfinoalanine decarboxylase can turn cysteine into hypotaurine and then taurine.[1] Alternately, the cysteine from the cystathionine gamma-lyase can be used by the enzymes glutamate–cysteine ligase (GCL) and glutathione synthetase (GSS) to produce glutathione.