Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension
- PMID: 30141981
- PMCID: PMC6297810
- DOI: 10.1152/ajpheart.00136.2018
Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension
Abstract
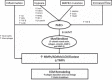
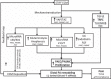
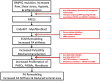
Pulmonary arterial hypertension (PAH) is characterized by remodeling of the extracellular matrix (ECM) of the pulmonary arteries with increased collagen deposition, cross-linkage of collagen, and breakdown of elastic laminae. Extracellular matrix remodeling occurs due to an imbalance in the proteolytic enzymes, such as matrix metalloproteinases, elastases, and lysyl oxidases, and tissue inhibitor of matrix metalloproteinases, which, in turn, results from endothelial cell dysfunction, endothelial-to-mesenchymal transition, and inflammation. ECM remodeling and pulmonary vascular stiffness occur early in the disease process, before the onset of the increase in the intimal and medial thickness and pulmonary artery pressure, suggesting that the ECM is a cause rather than a consequence of distal pulmonary vascular remodeling. ECM remodeling and increased pulmonary arterial stiffness promote proliferation of pulmonary vascular cells (endothelial cells, smooth muscle cells, and adventitial fibroblasts) through mechanoactivation of various signaling pathways, including transcriptional cofactors YAP/TAZ, transforming growth factor-β, transient receptor potential channels, Toll-like receptor, and NF-κB. Inhibition of ECM remodeling and mechanotransduction prevents and reverses experimental pulmonary hypertension. These data support a central role for ECM remodeling in the pathogenesis of the PAH, making it an attractive novel therapeutic target.
Keywords: collagen; compliance; mechanotransduction; right ventricle; stiffness.
Figures
Comment in
-
The extracellular matrix in early and advanced pulmonary arterial hypertension.Am J Physiol Heart Circ Physiol. 2018 Dec 1;315(6):H1684-H1686. doi: 10.1152/ajpheart.00620.2018. Epub 2018 Sep 28. Am J Physiol Heart Circ Physiol. 2018. PMID: 30265148 No abstract available.
Similar articles
-
Lysyl oxidases play a causal role in vascular remodeling in clinical and experimental pulmonary arterial hypertension.Arterioscler Thromb Vasc Biol. 2014 Jul;34(7):1446-58. doi: 10.1161/ATVBAHA.114.303534. Epub 2014 May 15. Arterioscler Thromb Vasc Biol. 2014. PMID: 24833797
-
Pathobiology of pulmonary arterial hypertension: understanding the roads less travelled.Eur Respir Rev. 2017 Dec 20;26(146):170093. doi: 10.1183/16000617.0093-2017. Print 2017 Dec 31. Eur Respir Rev. 2017. PMID: 29263173 Free PMC article. Review.
-
Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension.J Clin Invest. 2016 Sep 1;126(9):3313-35. doi: 10.1172/JCI86387. Epub 2016 Aug 22. J Clin Invest. 2016. PMID: 27548520 Free PMC article.
-
Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats.Respir Res. 2014 Nov 25;15:148. doi: 10.1186/s12931-014-0148-4. Respir Res. 2014. PMID: 25420924 Free PMC article.
-
Inflammation in pulmonary artery hypertension.Vascul Pharmacol. 2019 Jul-Aug;118-119:106562. doi: 10.1016/j.vph.2019.05.002. Epub 2019 May 18. Vascul Pharmacol. 2019. PMID: 31112745 Review. No abstract available.
Cited by
-
Utility of Serum Matrix Metalloproteinase-7 as a Biomarker in Cholestatic Infants with Congenital Heart Disease.Pediatr Cardiol. 2024 Nov 5. doi: 10.1007/s00246-024-03696-2. Online ahead of print. Pediatr Cardiol. 2024. PMID: 39499285
-
Inhibiting YAP1 reduced abdominal aortic aneurysm formation by suppressing adventitial fibroblast phenotype transformation and migration.J Cell Mol Med. 2024 Nov;28(21):e70159. doi: 10.1111/jcmm.70159. J Cell Mol Med. 2024. PMID: 39495769 Free PMC article.
-
Utility of Serum Matrix Metalloproteinase-7 as a Biomarker in Cholestatic Infants with Congenital Heart Disease.Res Sq [Preprint]. 2024 Oct 18:rs.3.rs-5004969. doi: 10.21203/rs.3.rs-5004969/v1. Res Sq. 2024. Update in: Pediatr Cardiol. 2024 Nov 5. doi: 10.1007/s00246-024-03696-2 PMID: 39483898 Free PMC article. Updated. Preprint.
-
Proteomic Signatures of Right Ventricular Outcomes in Pulmonary Arterial Hypertension.Circ Heart Fail. 2024 Nov;17(11):e012067. doi: 10.1161/CIRCHEARTFAILURE.124.012067. Epub 2024 Oct 22. Circ Heart Fail. 2024. PMID: 39435559
-
New insights into the mechanisms of the extracellular matrix and its therapeutic potential in anaplastic thyroid carcinoma.Sci Rep. 2024 Sep 9;14(1):20977. doi: 10.1038/s41598-024-72020-y. Sci Rep. 2024. PMID: 39251678 Free PMC article. Review.
References
-
- Anand V, Roy SS, Archer SL, Weir EK, Garg SK, Duval S, Thenappan T. Trends and outcomes of pulmonary arterial hypertension-related hospitalizations in the United States: analysis of the nationwide inpatient sample database from 2001 through 2012. JAMA Cardiol 1: 1021–1029, 2016. doi:10.1001/jamacardio.2016.3591. - DOI - PubMed
-
- Anwar A, Li M, Frid MG, Kumar B, Gerasimovskaya EV, Riddle SR, McKeon BA, Thukaram R, Meyrick BO, Fini MA, Stenmark KR. Osteopontin is an endogenous modulator of the constitutively activated phenotype of pulmonary adventitial fibroblasts in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 303: L1–L11, 2012. doi:10.1152/ajplung.00050.2012. - DOI - PMC - PubMed
-
- Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 122: 164–172, 2010. doi:10.1161/CIRCULATIONAHA.109.898122. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

