Chlamydophila felis CF0218 is a novel TMH family protein with potential as a diagnostic antigen for diagnosis of C. felis infection
- PMID: 18768674
- PMCID: PMC2565923
- DOI: 10.1128/CVI.00134-08
Chlamydophila felis CF0218 is a novel TMH family protein with potential as a diagnostic antigen for diagnosis of C. felis infection
Abstract
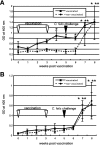
Chlamydophila felis is a causative agent of acute and chronic conjunctivitis and pneumonia in cats (feline chlamydiosis). Also, C. felis is a suspected zoonotic agent of such diseases as non-Chlamydia trachomatis conjunctivitis in humans, although this is controversial. At present, there is no serodiagnostic system that specifically detects C. felis infection conveniently. Current systems use antigens such as lipopolysaccharide that cross-react with all chlamydia species. In addition, it is difficult to distinguish between cats that are vaccinated with the commercial vaccine against C. felis and cats that are infected with C. felis. Here, we describe a new candidate diagnostic antigen for diagnosis of C. felis infection, CF0218, that was obtained by screening a genomic expression library of C. felis Fe/C-56 with C. felis-immunized serum. CF0218 was a putative transmembrane head (TMH) family protein with bilobed hydrophobic motifs at its N terminus, and orthologues of CF0218 were not found in the Chlamydophila pneumoniae or Chlamydia trachomatis genomes. The recombinant CF0218 was not recognized by antiserum against C. trachomatis, suggesting that CF0218 is C. felis specific. CF0218 transcription during the course of C. felis infection was confirmed by reverse transcription-PCR. By indirect immunofluorescence analysis, CF0218 was colocalized with the C. felis-formed inclusion bodies in the infected cells. The antibody response against CF0218 was elevated following C. felis infection but not by vaccination in experimentally vaccinated and infected cats. These results suggest that CF0218, a novel TMH family protein of C. felis, possesses potential as a C. felis infection-specific diagnostic antigen.
Figures




Similar articles
-
Using CF0218-ELISA to distinguish Chlamydophila felis-infected cats from vaccinated and uninfected domestic cats.Vet Microbiol. 2010 Dec 15;146(3-4):366-70. doi: 10.1016/j.vetmic.2010.05.026. Epub 2010 May 27. Vet Microbiol. 2010. PMID: 21095510
-
Polymorphic membrane proteins 1 and 7 from Chlamydophila felis are significant immunodominant proteins.Vet Microbiol. 2010 Aug 26;144(3-4):415-21. doi: 10.1016/j.vetmic.2010.02.021. Epub 2010 Feb 18. Vet Microbiol. 2010. PMID: 20227201
-
Feline chlamydiosis.Clin Tech Small Anim Pract. 2005 May;20(2):129-34. doi: 10.1053/j.ctsap.2004.12.018. Clin Tech Small Anim Pract. 2005. PMID: 15948428 Review.
-
Evaluation of cytologic findings in feline conjunctivitis.Vet Clin Pathol. 2012 Jun;41(2):283-90. doi: 10.1111/j.1939-165X.2012.00423.x. Epub 2012 May 2. Vet Clin Pathol. 2012. PMID: 22551068
-
Chlamydophila felis infection. ABCD guidelines on prevention and management.J Feline Med Surg. 2009 Jul;11(7):605-9. doi: 10.1016/j.jfms.2009.05.009. J Feline Med Surg. 2009. PMID: 19481040 Free PMC article. Review.
Cited by
-
The parasite Schistocephalus solidus secretes proteins with putative host manipulation functions.Parasit Vectors. 2021 Aug 28;14(1):436. doi: 10.1186/s13071-021-04933-w. Parasit Vectors. 2021. PMID: 34454597 Free PMC article.
-
Detection of Chlamydiaceae and Chlamydia-like organisms on the ocular surface of children and adults from a trachoma-endemic region.Sci Rep. 2018 May 9;8(1):7432. doi: 10.1038/s41598-018-23887-1. Sci Rep. 2018. PMID: 29743637 Free PMC article.
-
The Chlamydia psittaci genome: a comparative analysis of intracellular pathogens.PLoS One. 2012;7(4):e35097. doi: 10.1371/journal.pone.0035097. Epub 2012 Apr 10. PLoS One. 2012. PMID: 22506068 Free PMC article.
-
Multi-genome identification and characterization of chlamydiae-specific type III secretion substrates: the Inc proteins.BMC Genomics. 2011 Feb 16;12:109. doi: 10.1186/1471-2164-12-109. BMC Genomics. 2011. PMID: 21324157 Free PMC article.
References
-
- Azuma, Y., H. Hirakawa, A. Yamashita, Y. Cai, M. A. Rahman, H. Suzuki, S. Mitaku, H. Toh, S. Goto, T. Murakami, K. Sugi, H. Hayashi, H. Fukushi, M. Hattori, S. Kuhara, and M. Shirai. 2006. Genome sequence of the cat pathogen, Chlamydophila felis. DNA Res. 13:15-23. - PubMed
-
- Baker, J. A. 1942. A virus obtained from a pneumonia of cats and its possible relation to the cause of atypical pneumonia in man. Science 96:475-476. - PubMed
-
- Bannantine, J. P., R. S. Griffiths, W. Viratyosin, W. J. Brown, and D. D. Rockey. 2000. A secondary structure motif predictive of protein localization to the chlamydial inclusion membrane. Cell. Microbiol. 2:35-47. - PubMed
-
- Bannantine, J. P., D. D. Rockey, and T. Hackstadt. 1998. Tandem genes of Chlamydia psittaci that encode proteins localized to the inclusion membrane. Mol. Microbiol. 28:1017-1026. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

