Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates
- PMID: 18487200
- PMCID: PMC2475701
- DOI: 10.1074/jbc.M709319200
Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates
Abstract
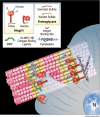
Type I collagen, the predominant protein of vertebrates, polymerizes with type III and V collagens and non-collagenous molecules into large cable-like fibrils, yet how the fibril interacts with cells and other binding partners remains poorly understood. To help reveal insights into the collagen structure-function relationship, a data base was assembled including hundreds of type I collagen ligand binding sites and mutations on a two-dimensional model of the fibril. Visual examination of the distribution of functional sites, and statistical analysis of mutation distributions on the fibril suggest it is organized into two domains. The "cell interaction domain" is proposed to regulate dynamic aspects of collagen biology, including integrin-mediated cell interactions and fibril remodeling. The "matrix interaction domain" may assume a structural role, mediating collagen cross-linking, proteoglycan interactions, and tissue mineralization. Molecular modeling was used to superimpose the positions of functional sites and mutations from the two-dimensional fibril map onto a three-dimensional x-ray diffraction structure of the collagen microfibril in situ, indicating the existence of domains in the native fibril. Sequence searches revealed that major fibril domain elements are conserved in type I collagens through evolution and in the type II/XI collagen fibril predominant in cartilage. Moreover, the fibril domain model provides potential insights into the genotype-phenotype relationship for several classes of human connective tissue diseases, mechanisms of integrin clustering by fibrils, the polarity of fibril assembly, heterotypic fibril function, and connective tissue pathology in diabetes and aging.
Figures





Similar articles
-
Collagen Structure-Function Mapping Informs Applications for Regenerative Medicine.Bioengineering (Basel). 2020 Dec 29;8(1):3. doi: 10.3390/bioengineering8010003. Bioengineering (Basel). 2020. PMID: 33383610 Free PMC article. Review.
-
The fibril-associated collagen IX provides a novel mechanism for cell adhesion to cartilaginous matrix.J Biol Chem. 2004 Dec 3;279(49):51677-87. doi: 10.1074/jbc.M409412200. Epub 2004 Sep 21. J Biol Chem. 2004. PMID: 15383545
-
Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen.J Biol Chem. 2002 Feb 8;277(6):4223-31. doi: 10.1074/jbc.M110709200. Epub 2001 Nov 9. J Biol Chem. 2002. PMID: 11704682
-
Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly.Micron. 2001 Apr;32(3):223-37. doi: 10.1016/s0968-4328(00)00043-3. Micron. 2001. PMID: 11006503 Review.
-
The collagens of articular cartilage.Semin Arthritis Rheum. 1991 Dec;21(3 Suppl 2):2-11. doi: 10.1016/0049-0172(91)90035-x. Semin Arthritis Rheum. 1991. PMID: 1796302 Review.
Cited by
-
Profiling of collagen and extracellular matrix deposition from cell culture using in vitro ExtraCellular matrix mass spectrometry imaging (ivECM-MSI).Matrix Biol Plus. 2024 Sep 25;24:100161. doi: 10.1016/j.mbplus.2024.100161. eCollection 2024 Dec. Matrix Biol Plus. 2024. PMID: 39435160 Free PMC article.
-
A yeast two-hybrid system to obtain triple-helical ligands from combinatorial random peptide libraries.J Biol Chem. 2024 Sep 19;300(11):107794. doi: 10.1016/j.jbc.2024.107794. Online ahead of print. J Biol Chem. 2024. PMID: 39305955 Free PMC article.
-
Contrast enhanced photoacoustic detection of fibrillar collagen in the near infrared region-I.Nanoscale Adv. 2024 Jun 7;6(14):3655-3667. doi: 10.1039/d4na00204k. eCollection 2024 Jul 9. Nanoscale Adv. 2024. PMID: 38989511 Free PMC article.
-
The Spatial Extracellular Proteomic Tumor Microenvironment Distinguishes Molecular Subtypes of Hepatocellular Carcinoma.J Proteome Res. 2024 Sep 6;23(9):3791-3805. doi: 10.1021/acs.jproteome.4c00099. Epub 2024 Jul 9. J Proteome Res. 2024. PMID: 38980715 Free PMC article.
-
Extracellular Microenvironment Alterations in Ductal Carcinoma In Situ and Invasive Breast Cancer Pathologies by Multiplexed Spatial Proteomics.Int J Mol Sci. 2024 Jun 19;25(12):6748. doi: 10.3390/ijms25126748. Int J Mol Sci. 2024. PMID: 38928454 Free PMC article.
References
-
- Piez, K. A., and Reddi, A. H. (1984) Extracellular Matrix Biochemistry, Elsevier, New York
-
- Ayad, S., Boot-handford, R. P., Humpries, M. J., Kadler, K. E., and Shuttleworth, C. A. (1998) The Extracellular Matrix Facts Book, 2nd Ed., Academic Press, San Diego
-
- Prockop, D. J., and Kivirikko, K. I. (1995) Annu. Rev. Biochem. 64 403-434 - PubMed
-
- Kadler, K. E., Baldock, C., Bella, J., and Boot-Handford, R. P. (2007) J. Cell Sci. 120 1955-1958 - PubMed
-
- Marini, J. C., Forlino, A., Cabral, W. A., Barnes, A. M., San Antonio, J. D., Milgrom, S., Hyland, J. C., Korkko, J., Prockop, D. J., De Paepe, A., Coucke, P., Symoens, S., Glorieux, F. H., Roughley, P. J., Lund, A. M., Kuurila-Svahn, K., Hartikka, H., Cohn, D. H., Krakow, D., Mottes, M., Schwarze, U., Chen, D., Yang, K., Kuslich, C., Troendle, J., Dalgleish, R., and Byers, P. H. (2007) Hum. Mutat. 28 209-221 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 RR008630-127842/RR/NCRR NIH HHS/United States
- P41 GM103622/GM/NIGMS NIH HHS/United States
- AR 048544/AR/NIAMS NIH HHS/United States
- RR 08630/RR/NCRR NIH HHS/United States
- R01 AR048544-04/AR/NIAMS NIH HHS/United States
- R01 AR048544/AR/NIAMS NIH HHS/United States
- AR 049604/AR/NIAMS NIH HHS/United States
- P41 RR008630/RR/NCRR NIH HHS/United States
- P41 RR008630-08/RR/NCRR NIH HHS/United States
- R21 AR049604-03/AR/NIAMS NIH HHS/United States
- R01 HL053590-08/HL/NHLBI NIH HHS/United States
- HL 053590/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

