Photobiomodulation as part of multimodal analgesia to improve pain relief and wound healing after elective caesarean section: A protocol for randomized controlled trial
- PMID: 39724020
- PMCID: PMC11670968
- DOI: 10.1371/journal.pone.0314010
Photobiomodulation as part of multimodal analgesia to improve pain relief and wound healing after elective caesarean section: A protocol for randomized controlled trial
Abstract
Background: Caesarean section (CS) is the most common inpatient surgical procedure performed in Canada. CS is known to cause moderate-to-severe pain, which is suggested to be associated with postpartum depression and persistent pain. Existing limitations in multimodal analgesia and conscious attempts to avoid opioids highlight the need for non-pharmacological strategies. Photobiomodulation therapy (PBMT) uses light-emitting diode (LED) and laser and has suggested potential for improving pain control and wound healing. This study aims to evaluate the effectiveness of PBMT as part of existing multimodal analgesia after elective CSs.
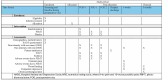
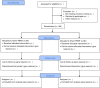
Methods: This placebo-controlled, two-arm, multi-centre, parallel-design randomized controlled trial includes women aged ≥16 years with planned CS under spinal anesthesia (Clinical Trials Registration: NCT05738239). Patients will be randomized post-CS to intervention (n = 90) or placebo (n = 90). Study interventions will be carried out using equipment supported by Meditech International Incorporated (approved by Health Canada for pain relief). Patients will receive a maximum of 5 post-surgical treatment sessions of active PBMT (intervention: LED therapy: DUO 240 [red at 660nm and near-infrared at 840nm] applied parallel to the abdominal incision scar, followed by BIOFLEX LDR-100 laser probe (660nm red light) and the LD1-200 laser probe (825nm near-infrared light), applied at the incision wound edges) or non-effective doses of LED array and laser therapy (placebo), 4-6 hrs post-CS, and at 8am and 7pm of postoperative days 1 and 2. Patients, research assistants involved in patient recruitment and follow-up, health care providers, and data analysts will be blinded. All patients will have access to routine multimodal analgesia. Patients will be followed up in hospital on the evening of surgery and on postoperative days 1 and 2 (morning, noon, and evening); at 6 weeks; and at 3 months by telephone. Primary outcome is pain intensity with movement (elicited by asking the patient to move from supine to sitting position) using 0-10 Numerical Rating Scale (0 = no pain, 10 = worst possible pain).
Significance: The results of this study may result in improved pain control, maternal satisfaction and wound healing; decrease the use of perioperative opioids; potentially decrease the incidence of postpartum depression and persistent pain; and overall lead to better postoperative outcomes thereby decreasing healthcare costs.
Copyright: © 2024 Khaled et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Canadian Institute for Health Information. Inpatient Hospitalization, Surgery and Newborn Statistics, 2020–2021. Ottawa, ON: CIHI; 2022.
-
- Guevara J, Carvalho JCA, Downey K, Ye XY, Sharkey AM, Arzola C. Predicting pain after Cesarean delivery: pressure algometry, temporal summation, three-item questionnaire. Can J Anaesth. 2021; 68: 1802–1810. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical