The OVAREX study: Establishment of ex vivo ovarian cancer models to validate innovative therapies and to identify predictive biomarkers
- PMID: 38849726
- PMCID: PMC11157894
- DOI: 10.1186/s12885-024-12429-w
The OVAREX study: Establishment of ex vivo ovarian cancer models to validate innovative therapies and to identify predictive biomarkers
Abstract
Background: Ovarian cancer is the first cause of death from gynecological malignancies mainly due to development of chemoresistance. Despite the emergence of PARP inhibitors, which have revolutionized the therapeutic management of some of these ovarian cancers, the 5-year overall survival rate remains around 45%. Therefore, it is crucial to develop new therapeutic strategies, to identify predictive biomarkers and to predict the response to treatments. In this context, functional assays based on patient-derived tumor models could constitute helpful and relevant tools for identifying efficient therapies or to guide clinical decision making.
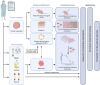
Method: The OVAREX study is a single-center non-interventional study which aims at investigating the feasibility of establishing in vivo and ex vivo models and testing ex vivo models to predict clinical response of ovarian cancer patients. Patient-Derived Xenografts (PDX) will be established from tumor fragments engrafted subcutaneously into immunocompromised mice. Explants will be generated by slicing tumor tissues and Ascites-Derived Spheroids (ADS) will be isolated following filtration of ascites. Patient-derived tumor organoids (PDTO) will be established after dissociation of tumor tissues or ADS, cell embedding into extracellular matrix and culture in specific medium. Molecular and histological characterizations will be performed to compare tumor of origin and paired models. Response of ex vivo tumor-derived models to conventional chemotherapy and PARP inhibitors will be assessed and compared to results of companion diagnostic test and/or to the patient's response to evaluate their predictive value.
Discussion: This clinical study aims at generating PDX and ex vivo models (PDTO, ADS, and explants) from tumors or ascites of ovarian cancer patients who will undergo surgical procedure or paracentesis. We aim at demonstrating the predictive value of ex vivo models for their potential use in routine clinical practice as part of precision medicine, as well as establishing a collection of relevant ovarian cancer models that will be useful for the evaluation of future innovative therapies.
Trial registration: The clinical trial has been validated by local research ethic committee on January 25th 2019 and registered at ClinicalTrials.gov with the identifier NCT03831230 on January 28th 2019, last amendment v4 accepted on July 18, 2023.
Keywords: Explants; Ovarian cancer; Patient-derived tumor organoids; Patient-derived tumor xenografts; Predictive functional assays.; Spheroids.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The TRIPLEX study: use of patient-derived tumor organoids as an innovative tool for precision medicine in triple-negative breast cancer.BMC Cancer. 2023 Sep 19;23(1):883. doi: 10.1186/s12885-023-11362-8. BMC Cancer. 2023. PMID: 37726786 Free PMC article.
-
ORGAVADS: establishment of tumor organoids from head and neck squamous cell carcinoma to assess their response to innovative therapies.BMC Cancer. 2023 Mar 9;23(1):223. doi: 10.1186/s12885-023-10692-x. BMC Cancer. 2023. PMID: 36894916 Free PMC article.
-
Comparative analysis of response to treatments and molecular features of tumor-derived organoids versus cell lines and PDX derived from the same ovarian clear cell carcinoma.J Exp Clin Cancer Res. 2023 Oct 7;42(1):260. doi: 10.1186/s13046-023-02809-8. J Exp Clin Cancer Res. 2023. PMID: 37803448 Free PMC article.
-
The role of patient-derived ovarian cancer organoids in the study of PARP inhibitors sensitivity and resistance: from genomic analysis to functional testing.J Exp Clin Cancer Res. 2021 Oct 26;40(1):338. doi: 10.1186/s13046-021-02139-7. J Exp Clin Cancer Res. 2021. PMID: 34702316 Free PMC article. Review.
-
Molecular and clinical implementations of ovarian cancer mouse avatar models.Chin Clin Oncol. 2015 Sep;4(3):30. doi: 10.3978/j.issn.2304-3865.2015.04.01. Chin Clin Oncol. 2015. PMID: 26408297 Free PMC article. Review.
References
-
- Torres D, Wang C, Kumar A, Bakkum-Gamez JN, Weaver AL, McGree ME, et al. Factors that influence survival in high-grade serous ovarian cancer: a complex relationship between molecular subtype, disease dissemination, and operability. Gynecol Oncol août. 2018;150(2):227–32. doi: 10.1016/j.ygyno.2018.06.002. - DOI - PMC - PubMed
-
- Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 29 déc. 2011;365(26):2484–96. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical