Effect of hypoxia‑HIF‑1��‑periostin axis in thyroid cancer
- PMID: 38391012
- PMCID: PMC10915707
- DOI: 10.3892/or.2024.8716
Effect of hypoxia‑HIF‑1α‑periostin axis in thyroid cancer
Abstract
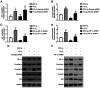
The incidence of thyroid carcinoma (TC) has exhibited a rapid increase in recent years. A proportion of TCs exhibit aggressive behavior. The present study aimed to investigate the potential role of hypoxia‑hypoxia inducible factor 1 subunit α (HIF‑1α)‑periostin axis in the progression of TC. The upregulation of periostin and HIF‑1α expression levels was detected in 95 clinical TC tissues as compared with normal thyroid tissues. Hypoxia promoted the viability and invasion of TC cells and this effect was inhibited by the downregulation of periostin. Hypoxia also induced the Warburg effect in TC and this effect was inhibited by the silencing of periostin. Further investigations revealed that hypoxia activated HIF‑1α, which in turn regulated the expression of periostin. Immunoprecipitation and dual luciferase reporter assays demonstrated that HIF‑1α upregulated the expression of periostin by binding to the promoter of periostin. On the whole, these findings suggest the existence of a hypoxia‑HIF‑1α‑periostin axis in TC and indicate the role of this axis in the progression of TC.
Keywords: Warburg effect; hypoxia; hypoxia inducible factor 1 subunit alpha; periostin; thyroid cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Lysine-Specific Demethylase 1 Affects the Progression of Papillary Thyroid Carcinoma via HIF1α and microRNA-146a.J Clin Endocrinol Metab. 2020 Jul 1;105(7):dgaa182. doi: 10.1210/clinem/dgaa182. J Clin Endocrinol Metab. 2020. PMID: 32303750
-
Hypoxia promotes thyroid cancer progression through HIF1α/FGF11 feedback loop.Exp Cell Res. 2022 Jul 1;416(1):113159. doi: 10.1016/j.yexcr.2022.113159. Epub 2022 Apr 14. Exp Cell Res. 2022. PMID: 35430272
-
Periostin contributes to arsenic trioxide resistance in hepatocellular carcinoma cells under hypoxia.Biomed Pharmacother. 2017 Apr;88:342-348. doi: 10.1016/j.biopha.2017.01.052. Epub 2017 Jan 21. Biomed Pharmacother. 2017. PMID: 28119236
-
MiR-548c-3p suppressed the progression of papillary thyroid carcinoma via inhibition of the HIF1α-mediated VEGF signaling pathway.Eur Rev Med Pharmacol Sci. 2019 Aug;23(15):6570-6578. doi: 10.26355/eurrev_201908_18543. Eur Rev Med Pharmacol Sci. 2019. PMID: 31378898
-
Effect of perioperative treatment with a hypoxia-inducible factor-1-alpha inhibitor in an orthotopic surgical mouse model of thyroid cancer.Anticancer Res. 2015 Apr;35(4):2049-54. Anticancer Res. 2015. PMID: 25862859
Cited by
-
A Comprehensive Review of Protein Biomarkers for Invasive Lung Cancer.Curr Oncol. 2024 Aug 23;31(9):4818-4854. doi: 10.3390/curroncol31090360. Curr Oncol. 2024. PMID: 39329988 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

