Amphetamine Derivatives as Monoamine Oxidase Inhibitors
- PMID: 32038257
- PMCID: PMC6989591
- DOI: 10.3389/fphar.2019.01590
Amphetamine Derivatives as Monoamine Oxidase Inhibitors
Abstract
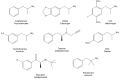
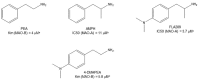
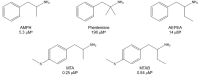
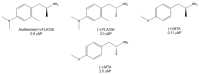
Amphetamine and its derivatives exhibit a wide range of pharmacological activities, including psychostimulant, hallucinogenic, entactogenic, anorectic, or antidepressant effects. The mechanisms of action underlying these effects are usually related to the ability of the different amphetamines to interact with diverse monoamine transporters or receptors. Moreover, many of these compounds are also potent and selective monoamine oxidase inhibitors. In the present work, we review how structural modifications on the aromatic ring, the amino group and/or the aliphatic side chain of the parent scaffold, modulate the enzyme inhibitory properties of hundreds of amphetamine derivatives. Furthermore, we discuss how monoamine oxidase inhibition might influence the pharmacology of these compounds.
Keywords: amphetamine derivatives; dopamine transporter; monoamine oxidase; monoamine oxidase-A; norepinephrine transporter; serotonin syndrome; serotonin transporter.
Copyright © 2020 Reyes-Parada, Iturriaga-Vasquez and Cassels.
Figures


Similar articles
-
Monoamine oxidase inhibitory properties of some methoxylated and alkylthio amphetamine derivatives: structure-activity relationships.Biochem Pharmacol. 1997 Dec 15;54(12):1361-9. doi: 10.1016/s0006-2952(97)00405-x. Biochem Pharmacol. 1997. PMID: 9393679
-
Pharmacological profile of mephedrone analogs and related new psychoactive substances.Neuropharmacology. 2018 May 15;134(Pt A):4-12. doi: 10.1016/j.neuropharm.2017.07.026. Epub 2017 Jul 26. Neuropharmacology. 2018. PMID: 28755886
-
[INTERFERENCE BY D-AMPHETAMINE IN THE ACTION OF SOME MONOAMINE OXIDASE INHIBITORS].Boll Soc Ital Biol Sper. 1963 Nov 30;39:1381-4. Boll Soc Ital Biol Sper. 1963. PMID: 14123621 Italian. No abstract available.
-
MONOAMINE OXIDASE INHIBITORS.Anaesthesia. 1964 Jul;19:376-86. doi: 10.1111/j.1365-2044.1964.tb00392.x. Anaesthesia. 1964. PMID: 14174638 Review. No abstract available.
-
Pharmacologic profile of amphetamine derivatives at various brain recognition sites: selective effects on serotonergic systems.NIDA Res Monogr. 1989;94:240-58. NIDA Res Monogr. 1989. PMID: 2514364 Review.
Cited by
-
Applications and Potential of In Silico Approaches for Psychedelic Chemistry.Molecules. 2023 Aug 9;28(16):5966. doi: 10.3390/molecules28165966. Molecules. 2023. PMID: 37630218 Free PMC article. Review.
-
Many Drugs of Abuse May Be Acutely Transformed to Dopamine, Norepinephrine and Epinephrine In Vivo.Int J Mol Sci. 2021 Oct 2;22(19):10706. doi: 10.3390/ijms221910706. Int J Mol Sci. 2021. PMID: 34639047 Free PMC article. Review.
-
Synthesis, In Vitro Anti-Inflammatory Activity, and HRMS Analysis of New Amphetamine Derivatives.Molecules. 2022 Dec 24;28(1):151. doi: 10.3390/molecules28010151. Molecules. 2022. PMID: 36615344 Free PMC article.
-
Pharmacological Characterization of 4-Methylthioamphetamine Derivatives.Molecules. 2020 Nov 13;25(22):5310. doi: 10.3390/molecules25225310. Molecules. 2020. PMID: 33203055 Free PMC article.
-
Effects of Early Life Exposure to Sex Hormones on Neurochemical and Behavioral Responses to Psychostimulants in Adulthood: Implications in Drug Addiction.Int J Mol Sci. 2022 Jun 12;23(12):6575. doi: 10.3390/ijms23126575. Int J Mol Sci. 2022. PMID: 35743018 Free PMC article.
References
-
- Arai Y., Kim S. K., Kinemuchi H., Tadano T., Satoh S. E., Satoh N., et al. (1990). Inhibition of brain type A monoamine oxidase and 5-hydroxytryptamine uptake by two amphetamine metabolites, p-hydroxyamphetamine and p-hydroxynorephedrine. J. Neurochem. 55, 403–408. 10.1111/j.1471-4159.1990.tb04151.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

