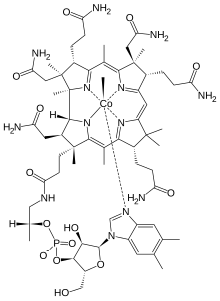
Methylcobalamin
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | oral,sublingual,injection. |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.033.200 |
| Chemical and physical data | |
| Formula | C63H91CoN13O14P |
| Molar mass | 1344.40 g/mol g·mol−1 |
| | |
Methylcobalamin is a cobalamin (MeCbl or MeB12) used in the treatment of peripheral neuropathy, diabetic neuropathy, and as a preliminary treatment for amyotrophic lateral sclerosis. It is a form of vitamin B12 and differs from cyanocobalamin in that the cyanide is replaced by a methyl group.[1] Methylcobalamin features an octahedral cobalt(III) centre. Methylcobalamin can as obtained as bright red crystals.[2]
From the perspective of coordination chemistry, methylcobalamin is notable as a rare example of an enzyme that contains metal-alkyl bonds. Nickel-methyl intermediates have been proposed for the final step of methanogenesis.
Production
Methylcobalamin can be produced in the laboratory by reducing cyanocobalamin with sodium borohydride in alkaline solution, followed by the addition of methyl iodide.[2]
Functions
This vitamer is one of two active coenzymes used by vitamin B12-dependent enzymes and is the specific vitamin B12 form used by 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR), also known as methionine synthase. In physiological terms, it is equivalent to vitamin B12, e.g. for addressing pathologies arising from a lack of vitamin B12, such as pernicious anemia.
Role in the environment
Methylcobalamin is produced by some bacteria. It plays an important role in the environment. In the environment, it is responsible for the biomethylation of certain heavy metals. For example, the highly toxic methylmercury is produced by the action of methylcobalamin.[3]
See also
References
- ^ L. R. McDowell, Vitamins in animal and human nutrition
- ^ a b David Dophin. Preparation of the Reduced Forms of Vitamin B12 and of Some Analogs of the Vitamin B12 Coenzyme Containing a Cobalt-Carbon Bond. D.B. McCormick and L.D. Wright, Eds. 1971;Vol. XVIII:34-54.
- ^ Zenon Schneider, Andrzej Stroiński, Comprehensive B12: Chemistry, Biochemistry, Nutrition, Ecology, Medicine
