Aspartame
| Aspartame | |
|---|---|
| Chemical name | N-L-α-Aspartyl-L-phenylalanine 1-methyl ester |
| Chemical formula | C14H18N2O5 |
| Molecular mass | 294.30 g/mol |
| Melting point | 246 - 247 °C |
| CAS number | 22839-47-0 |
| SMILES | [NH3+][C@@H](CC([O-])=O)C(N[C@@H] (CC1=CC=CC=C1)C(OC)=O)=O |
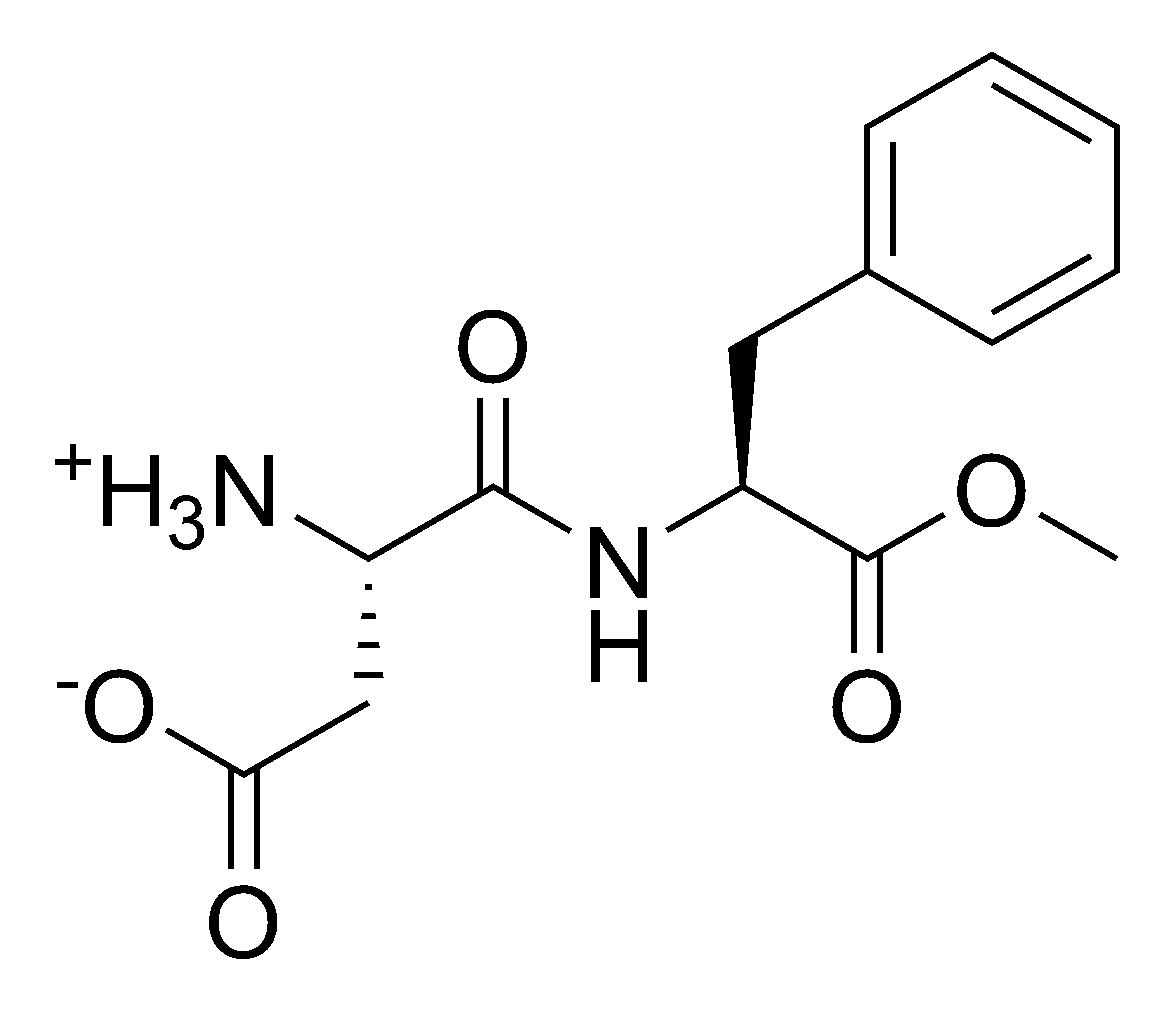
| |
Aspartame is the name for the artificial, non-carbohydrate sweetener, Aspartyl-phenylalanine-1-methyl ester; i.e. the methyl ester of the dipeptide of the amino acids aspartic acid and phenylalanine.
It is 160 times sweeter than sugar (sucrose or saccharose), and is marketed under a number of trademarked names, such as "NutraSweet", "Equal", and "Canderel". In the United States aspartame is often found in restaurants in blue packets. In the European Union it is also known under the E number (additive code) E951. It is a common sweetener in prepared foods, particularly soft drinks. Aspartame is one of the sugar substitutes used by diabetics. Products containing aspartame usually have a warning label that they contain phenylalanine, in compliance with U.S. FDA guidelines. Phenylalanine, a natural amino acid found in many foods, is deleterious only to sufferers of the genetic disorder phenylketonuria. Aspartame, being a peptide, breaks down into its constituent amino acids when heated in the presence of water and acids. Therefore, it is unsuitable for use in baking. However, it is commonly used in diet soft drinks such as Diet Coke or Diet Pepsi, or to sweeten coffee and tea. Aspartame has virtually zero nutritional value, which makes it a good substitute for people trying to avoid calories from sugar intake.
Some find the taste of Aspartame very unpleasant. It is thought that this taste response is genetic in nature, but so far no studies have been done.
Health effects
Discovery and approval
Aspartame was discovered in 1965 by James M. Schlatter at the G.D. Searle company (later purchased by Monsanto). Some initial safety testing suggested that aspartame might cause brain tumors in rats; as a result, the additive was held up in the United States for many years in the Food and Drug Administration's approval process. In 1980, the FDA convened a Public Board of Inquiry (PBOI) consisting of independent advisors charged with examining the purported relationship between aspartame and brain cancer.
The PBOI concluded that aspartame did not cause brain damage, but recommended against approving aspartame at that time, citing unanswered questions about cancer in laboratory rats. In 1981, FDA Commissioner Arthur Hull Hayes, newly appointed by President Ronald Reagan, approved aspartame as a food additive, citing data from a Japanese study that had not been available to the members of the PBOI. [1]
Purported health effects
Since the FDA approved aspartame for consumption, some researchers have suggested that a rise in brain tumor rates in the United States may be at least partially related to the increasing availability and consumption of aspartame. [2] However, more recent research has failed to find any link between aspartame and cancer or other health problems. [3], [4]
It has been suggested that aspartame might be a neurotoxin [5], since one of its ingredients is methanol (wood alcohol), that is converted in the body to formaldehyde. As a result, in September of 2004, a RICO lawsuit was filed against Nutrasweet and the Monsanto Chemical Company for producing aspartame.
One of the many hypotheses about the causes of Gulf war syndrome is that soldiers, after drinking gallons of soft drinks containing aspartame in the extreme heat, accumulated toxic doses of methanol, formaldehyde, diketopiperazine and formic acid from the breakdown of the sweetener into its component molecules. However, the symptoms do not greatly resemble those of classic methanol poisoning, and the body, in its normal metabolism, produces methanol in quantities comparable or greater than would be ingested via aspartame, so this theory does not have wide support.
Questions about aspartame frequently revolve around concerns of health conditions that are allegedly caused by the sweetener, including headaches, seizures, allergic reactions, and changes in mood or behaviour. Indeed, an e-mail has been circulating since 1998, claiming to be from a lecturer at an international conference on aspartame risks, listing various symptoms supposedly caused by the chemical. This email has generally been dismissed as inaccurate, and there is no evidence that such a conference or other events recounted in the email actually occurred. [6] It is worth bearing in mind that aspartame is commonly found in soft drinks containing other components which may cause some side-effects, for example, caffeine.
The overwhelming body of scientific evidence clearly shows that aspartame, even in amounts many times greater than what people typically consume, is safe and not associated with adverse health effects. The U.S. Food and Drug Administration has investigated claims of ill effects since 1982 and has confirmed that there is no reasonable evidence of possible public health harm and no consistent or unique patterns of symptoms reported with respect to aspartame that can be causally linked to its use. [7]
Chemistry
Aspartame is the methyl ester of the dipeptide of the amino acids l-aspartic acid and l-phenylalanine. Under strongly acidic or alkaline conditions, aspartame first splits off methanol by hydrolysis. Under more severe conditions, the peptide bonds are also hydrolyzed, resulting in the free amino acids.
See also
External links
- FDA Article
- Aspartame.org Info from a low-calorie Food & Beverage Industry-supported organization
- aspartamekills.com Anti-Aspartame website
- Scientific Facts on Aspartame - A summary by GreenFacts of the Update on the Safety of Aspartame (PDF), a scientific consensus report published by the European Commission.
- Aspartame Warnings
- Aspartame Archives
- Criticism of CDC report findings
