CAPER, a novel regulator of human breast cancer progression
- PMID: 24621503
- PMCID: PMC4049962
- DOI: 10.4161/cc.28156
CAPER, a novel regulator of human breast cancer progression
Abstract
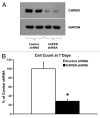
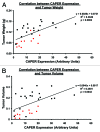
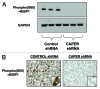
CAPER is an estrogen receptor (ER) co-activator that was recently shown to be involved in human breast cancer pathogenesis. Indeed, we reported increased expression of CAPER in human breast cancer specimens. We demonstrated that CAPER was undetectable or expressed at relatively low levels in normal breast tissue and assumed a cytoplasmic distribution. In contrast, CAPER was expressed at higher levels in ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC) specimens, where it assumed a predominantly nuclear distribution. However, the functional role of CAPER in human breast cancer initiation and progression remained unknown. Here, we used a lentiviral-mediated gene silencing approach to reduce the expression of CAPER in the ER-positive human breast cancer cell line MCF-7. The proliferation and tumorigenicity of MCF-7 cells stably expressing control or human CAPER shRNAs was then determined via both in vitro and in vivo experiments. Knockdown of CAPER expression significantly reduced the proliferation of MCF-7 cells in vitro. Importantly, nude mice injected with MCF-7 cells harboring CAPER shRNAs developed smaller tumors than mice injected with MCF-7 cells harboring control shRNAs. Mechanistically, tumors derived from mice injected with MCF-7 cells harboring CAPER shRNAs displayed reduced expression of the cell cycle regulators PCNA, MCM7, and cyclin D1, and the protein synthesis marker 4EBP1. In conclusion, knockdown of CAPER expression markedly reduced human breast cancer cell proliferation in both in vitro and in vivo settings. Mechanistically, knockdown of CAPER abrogated the activity of proliferative and protein synthesis pathways.
Keywords: CAPER; breast cancer; estrogen receptor; proliferation; tumor growth.
Figures



Similar articles
-
Development of CAPER peptides for the treatment of triple negative breast cancer.Cell Cycle. 2020 Feb;19(4):432-447. doi: 10.1080/15384101.2020.1711579. Epub 2020 Jan 13. Cell Cycle. 2020. PMID: 31931653 Free PMC article.
-
Nek2A contributes to tumorigenic growth and possibly functions as potential therapeutic target for human breast cancer.J Cell Biochem. 2012 Jun;113(6):1904-14. doi: 10.1002/jcb.24059. J Cell Biochem. 2012. PMID: 22234886
-
Maintenance of S-nitrosothiol homeostasis plays an important role in growth suppression of estrogen receptor-positive breast tumors.Breast Cancer Res. 2012 Dec 5;14(6):R153. doi: 10.1186/bcr3366. Breast Cancer Res. 2012. PMID: 23216744 Free PMC article.
-
Effect of specific silencing of EMMPRIN on the growth and cell cycle distribution of MCF-7 breast cancer cells.Genet Mol Res. 2015 Dec 2;14(4):15730-8. doi: 10.4238/2015.December.1.24. Genet Mol Res. 2015. PMID: 26634540
-
The molecular characteristics and functional roles of microspherule protein 1 (MCRS1) in gene expression, cell proliferation, and organismic development.Cell Cycle. 2023 Mar-Mar;22(6):619-632. doi: 10.1080/15384101.2022.2145816. Epub 2022 Nov 16. Cell Cycle. 2023. PMID: 36384428 Free PMC article. Review.
Cited by
-
Defects in Emerin-Nucleoskeleton Binding Disrupt Nuclear Structure and Promote Breast Cancer Cell Motility and Metastasis.Mol Cancer Res. 2021 Jul;19(7):1196-1207. doi: 10.1158/1541-7786.MCR-20-0413. Epub 2021 Mar 26. Mol Cancer Res. 2021. PMID: 33771882 Free PMC article.
-
Distinct prostate cancer-related mRNA cargo in extracellular vesicle subsets from prostate cell lines.BMC Cancer. 2017 Feb 1;17(1):92. doi: 10.1186/s12885-017-3087-x. BMC Cancer. 2017. PMID: 28143451 Free PMC article.
-
RBM39 is a potential prognostic biomarker with functional significance in hepatocellular carcinoma.Transl Cancer Res. 2024 Apr 30;13(4):1606-1622. doi: 10.21037/tcr-23-2252. Epub 2024 Apr 12. Transl Cancer Res. 2024. PMID: 38737697 Free PMC article.
-
The transcription factor c-Jun inhibits RBM39 to reprogram pre-mRNA splicing during genotoxic stress.Nucleic Acids Res. 2022 Dec 9;50(22):12768-12789. doi: 10.1093/nar/gkac1130. Nucleic Acids Res. 2022. PMID: 36477312 Free PMC article.
-
Systematic pan-cancer analysis identifies RBM39 as an immunological and prognostic biomarker.J Cell Mol Med. 2022 Sep;26(18):4859-4871. doi: 10.1111/jcmm.17517. Epub 2022 Aug 21. J Cell Mol Med. 2022. PMID: 35989423 Free PMC article.
References
-
- Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15:17–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
