Abstract
Upon vascular injury, locally controlled haemostasis prevents life-threatening blood loss and ensures wound healing. Intracellular material derived from damaged cells at these sites will become exposed to blood components and could contribute to blood coagulation and pathological thrombus formation. So far, the functional and mechanistic consequences of this concept are not understood. Here, we present in vivo and in vitro evidence that different forms of eukaryotic and prokaryotic RNA serve as promoters of blood coagulation. Extracellular RNA was found to augment (auto-)activation of proteases of the contact phase pathway of blood coagulation such as factors XII and XI, both exhibiting strong RNA binding. Moreover, administration of exogenous RNA provoked a significant procoagulant response in rabbits. In mice that underwent an arterial thrombosis model, extracellular RNA was found associated with fibrin-rich thrombi, and pretreatment with RNase (but not DNase) significantly delayed occlusive thrombus formation. Thus, extracellular RNA derived from damaged or necrotic cells particularly under pathological conditions or severe tissue damage represents the long sought natural “foreign surface” and provides a procoagulant cofactor template for the factors XII/XI-induced contact activation/amplification of blood coagulation. Extracellular RNA thereby reveals a yet unrecognized target for antithrombotic intervention, using RNase or related therapeutic strategies.
Keywords: contact phase activation, thrombosis, RNase, vascular injury, wound healing
Vascular injury markedly alters the properties of the innermost cell lining of our blood vessels, the endothelium (1). Immediate platelet adherence and aggregation with concomitant release of their granula contents is followed by local activation of blood coagulation. Together, these events prevent life-threatening blood loss and invasion of microorganisms and ensure wound healing. Upon contact with plasma proteins, tissue factor (expressed in deeper layers of the vessel wall or in association with activated platelets) induces the formation of minute amounts of thrombin that in turn will amplify its further production through direct activation of coagulation factor XI (a proform of a serine protease) and the nonenzymatic protein cofactors VIII and V (2–4). Together with anionic glycerophospholipids that become exposed after platelet activation or cellular disruption, the subsequent calcium-dependent assembly of multicomponent protease complexes comprises the central aspect of the exploding coagulation enzyme cascade (5–7) that allows spatiotemporal fibrin formation for tight sealing of the wound, which is necessary for undisturbed tissue repair.
The initiation of blood coagulation in vitro can be mediated by artificial polyanionic surfaces, such as kaolin or glass (8, 9), and by collagen or glycosaminoglycans (10, 11). These reactions involve the “contact phase” proteins, including factors XII and XI, prekallikrein, and high-molecular-weight kininogen that are equipped with particular anion-binding exosites. A respective physiological in vivo correlate of these surface-mediated reactions is missing so far (8, 10). Thus, the contribution of the bloodborne contact phase proteins in the initiation and amplification of physiological blood coagulation is obscure and also disputed; patients with hereditary deficiencies in factor XII or kininogen do not suffer from bleeding tendencies (12). Nevertheless, the autonomous activation of factor XI by thrombin constitutes an essential trigger in the amplification and production phase for in vivo thrombin generation (13), and some patients with factor XI deficiency suffer from bleeding tendencies (14).
In a previous study with a polyanion-binding haemostasis factor, designated “factor VII-activating protease,” extracellular RNA was shown to serve as potent cofactor for the (auto-)activation of this proenzyme (15), and stable RNA-protein complexes were formed (16). These results prompted us to elucidate the coagulatory activities of cell-free extracellular nucleic acids, in particular RNA. It has been hypothesized that plasmatic nucleic acids might also have a biological activity (17), and the RNA-proteolipid complexes detected in the circulation of cancer patients were postulated to mediate host–tumor interactions (18). So far, however, no experimental approaches were undertaken to characterize the functional role of cell-free extracellular RNA in vascular medicine.
Here, we provide evidence in vitro and in vivo that extracellular nucleic acids, in particular RNA, serve to promote the activation of contact phase proteins. Based on the identified procoagulant activities of extracellular RNA in vivo, we further demonstrate that administration of RNase (but not DNase) in an arterial thrombosis model provides a new modality to delay thrombus formation and blood vessel occlusion. Our data are supported by a recent study in which factor XII-deficient mice were found to be protected against severe thrombus formation in different models of thromboembolism (19). Thus, under severe conditions of tissue damage, the contact phase system appears to be relevant for thrombus formation.
Results
Extracellular RNA, RNase, and Thrombus Formation in Vivo.
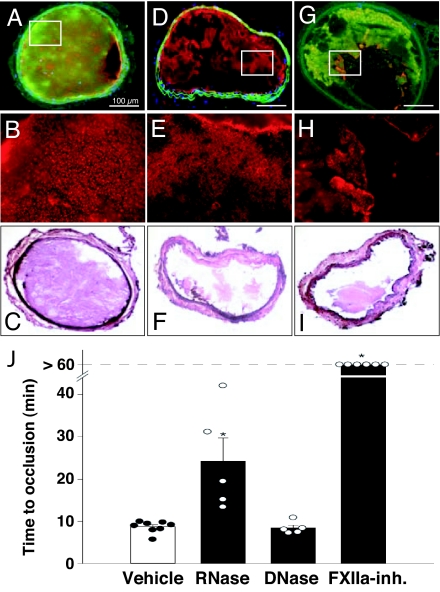
In an established mouse model of arterial thrombosis, local administration of ferric chloride to the adventitial vessel surface induced severe damage/necrosis of the intimal part of the vessel wall (20–22). In vehicle-treated animals, complete vessel occlusion by platelet-rich thrombus occurred after 545 ± 18 sec (Fig. 1 A–C and J). For the detection of extracellular RNA in situ, before FeCl3 exposure, RNASelect green fluorescent cell stain was injected into the left jugular vein of mice, and extracellular RNA-related staining was discernible in association with the occlusive arterial thrombus (Fig. 1A). No cell-free DNA was found associated with the thrombus material that was mainly composed of platelets in association with fibrin as shown in larger magnifications (Fig. 1 B, E, and H). Direct binding experiments in vitro revealed association of RNA with fibrillar collagens and fibrin but not with fibrinogen, indicating a specific interaction of the nucleic acid with thrombus and vessel matrix material (data not shown).
Fig. 1.
Association of extracellular RNA with thrombus formation and intervention with RNase in arterial thrombosis after vascular injury in vivo. Vascular injury of the mouse carotid artery was induced by local application of ferric chloride (A–I) in the absence (vehicle; A–C) or after pretreatment with RNase (D–F) or factor XIIa-inhibitor (G–I) as indicated (for details, see Materials and Methods). In all cases, before thrombus induction, an RNA-selective fluorescent green stain was administered (A, D, and G). Note the decoration of the thrombus by RNA in A and G, whereas pretreatment with RNase resulted in RNA hydrolysis and reduction of thrombus size (D) and pretreatment with FXIIa-inhibitor led to prevention of vessel occlusion without affecting RNA association with the thrombus (G). Postembedding staining against platelet glycoprotein Ib (red fluorescence in A, B, D, E, G, and H) indicates platelet-rich nature of thrombi. Counterstain for H&E is shown in C, F, and I for each group. (J) The time to thrombotic occlusion in the carotid artery downstream of the site of injury was assessed in vivo by video fluorescence microscopy in the absence (vehicle) or presence of RNase, DNase, or factor XIIa-inhibitor (FXIIa-Inh). Data represent mean ± SEM (n = 5–8 mice in each group). Circles represent results from individual experiments. ∗, P < 0.05 vs. vehicle and DNase (Mann–Whitney test). Representative images for each group are shown in SI Fig. 4.
In the RNase-treatment group mice, time to arterial occlusion was significantly prolonged to 1,457 ± 326 sec, and no RNA was found in association with the largely reduced thrombus material (Fig. 1 D–F and J), whereas administration of DNase had no influence [Fig. 1J and supporting information (SI) Fig. 4]. These results indicate that an arterial thrombus can harbor extracellular RNA and that counteracting RNase can exert a strong antithrombotic effect in vivo. For comparison, administration of a factor XIIa inhibitor had an even stronger vessel protective effect, and after 60 min, no occlusive thrombus was detectable (Fig. 1 G–J). As illustrated by corresponding intravital microscopy, RNase- and factor XIIa-inhibitor-dependent prolongation of time to full occlusion was due to the formation of large emboli leading to fragmentation of the thrombus and maintenance of residual blood flow, whereas in the vehicle and DNase treated groups, a full occlusive thrombus resulted in blood stasis (Fig. 1J and SI Fig. 4). Consequently, extracellular RNA may constitute the predominant nucleic acid species in vivo with putative procoagulant activity. In addition, tRNA from yeast or artificial RNA poly(inosinic acid)·poly(cytidylic acid) [poly(I·C)] injected into healthy rabbits induced a procoagulant response (SI Figs. 5 and 6). Pretreatment of RNA by RNase, RNase alone or the RNA equivalent dose of nucleotide monophosphates had no significant influence on the basal levels of coagulation parameters. Together, these data strongly indicate that natural as well as artifical RNA, the latter being known as structural analogue for double-stranded viral RNA (23), can facilitate blood coagulation in vivo.
Extracellular RNA Promotes (Auto-)Activation of Coagulation Proteases.
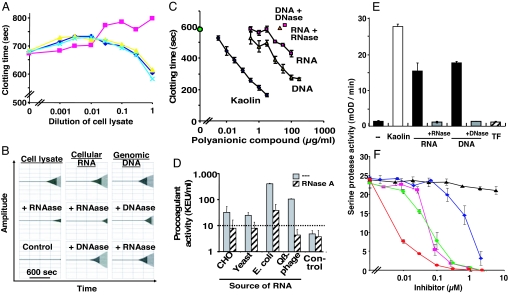
Cell lysate derived from cultured Chinese hamster ovary (CHO) cells (that do not express tissue factor) significantly augmented calcium-induced blood coagulation in normal plasma (Fig. 2A). Whereas pretreatment of cell lysate with DNase or antibodies to tissue factor had no influence, RNase abrogated the procoagulant activity, indicating that cell-derived RNA serves as procoagulant cofactor. Likewise, cell lysate and isolated nucleic acids (cellular RNA or genomic DNA) from CHO cells exhibited a significant augmentation of coagulation in whole blood by shortening the lag phase before coagulation began (Fig. 2B). Pretreatment of RNA or DNA with the respective nuclease resulted in a significant reduction or loss, respectively, of procoagulant activity. The nucleic acid-dependent induction of blood coagulation did not involve platelets, because RNA did not lead to platelet activation (as measured by P-selectin expression on washed platelets), and the nucleic acid did not induce platelet shape changes or aggregation (data not shown).
Fig. 2.
Procoagulant activity of cell lysate and extracellular nucleic acids. (A) The procoagulant activity of particle free cell lysate prepared from cultured CHO cells was analyzed in a turbidity coagulation assay in the absence (blue diamonds) or presence of RNase (purple squares), DNase (yellow triangles), or antitissue factor (green crosses). Data represent mean ± SD, n = 3 of a representative experiment of three. (B) The procoagulant activity of cell lysate as well as of isolated RNA or DNA as indicated was tested in whole blood thrombelastography, whereby the time period (lag phase) until thrombus formation occurs and its extent is measured (amplitude) (Upper Left, Right, and Center). Parallel samples were pretreated with RNase A or DNase as indicated in each case, and representative experiments of six are shown. (C) Compared with kaolin (diamonds) different concentrations of RNA (squares) and DNA (triangles) were tested for procoagulant activity in pooled normal human plasma by using turbidity clot-lysis assay. Buffer control is indicated by the green circle, and nuclease-pretreated RNA and DNA are indicated by the red square and triangle, respectively. Data represent the mean ± SD (n = 3) of one representative experiment of three. (D) Anticoagulated normal human plasma was supplemented with 10 μg/ml each of RNA prepared from CHO cells, tRNA from yeast, total RNA from E. coli, single-stranded RNA from Qβ-phage, or buffer (control) as indicated, and coagulation was initiated by recalcification. Both untreated (gray) and RNase-pretreated (hatched) samples were compared. Data are converted from coagulation times into kaolin-equivalent units per ml, as explained in Materials and Methods and represent mean ± SD (n = 3) of three individual experiments. (E) Kaolin (10 ng/ml, open bar) and untreated and nuclease-pretreated RNA or DNA (10 μg/ml each) as indicated were incubated with citrated normal human plasma, and an increase in serine protease activity was followed by a chomogenic substrate (S-2288) assay. In comparison, recombinant tissue factor (TF) (0.8 μg/ml, hatched bar) was analyzed. Data represent mean ± SD (n = 3) of of one representative experiment of three. (F) Using the same experimental protocol, serine protease activity of RNA-supplemented citrated plasma was followed in the absence or presence of increasing doses of aprotinin (blue diamonds), corn trypsin inhibitor (green circles), factor XIa inhibitor J545 (purple squares), kallikrein inhibitor J626 (red circles), or hirudin (filled triangles). Data represent mean ± SD (n = 3) of one representative experiment of three.
In the clinical laboratory, nonbiological polyanionic material such as kaolin is routinely used in diagnostic coagulation testing of patient plasma samples (24). Because nucleic acids appear to share the polyanionic character with kaolin, the comparative analysis with isolated RNA and DNA revealed that all three substances promoted blood coagulation in a concentration-dependent fashion in recalcified plasma in a comparable way (Fig. 2C). Although on a weight basis kaolin was the far most effective procoagulant compound because of its high polyanionic surface density, extracellular nucleic acids represent the natural analogue of kaolin.
To specify the type of RNA that contains procoagulant activity, equivalent doses of RNA from different sources were compared in a turbidity coagulation assay that measures the onset of fibrin formation in recalcified plasma, and results were expressed as “kaolin-equivalent units.” Eukaryotic ribosomal RNA isolated from CHO cells, transfer-RNA from yeast, Escherichia coli RNA, or single-stranded RNA from Qβ-phage all exhibited an appreciable procoagulant activity that was (except for E. coli RNA) abrogated by pretreatment with RNase A (Fig. 2D). Also, viral RNA from hepatitis C virus or picornavirus exhibited a comparable potent procoagulant activity (data not shown).
Binding to and Activation of Contact Phase Proteins by Nucleic Acids.
Isolated nucleic acids were tested for activation of contact phase proteins in citrated plasma and were found to induce a significant (kaolin-like) 15- to 20-fold elevation of serine protease activity that could be prevented by preincubation with the respective nuclease (Fig. 2E). As expected, tissue factor had no effect on induction of serine protease activity. In RNA-supplemented citrated plasma, both the broad spectrum inhibitor aprotinin and specific inhibitors against factor XIIa (corn trypsin inhibitor) or synthetic inhibitors against factor XIa and kallikrein were equally effective to inhibit RNA-induced serine protease activity completely (Fig. 2F). These results were corroborated by thrombelastographic and clotting assays in whole blood or plasma, respectively (SI Fig. 7), and underline our proposition that nucleic acids substantially promote induction/elevation of serine protease activity related to enzymes of the contact phase of blood coagulation. Analogously, these proteases were activated by artificial RNA (data not shown), indicative for the highly specific nature of nucleic acid material to interact with these plasma proteins.
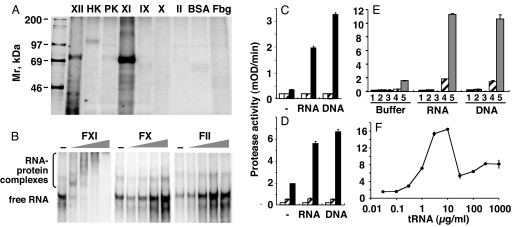
After binding and crosslinking of radiolabeled hepatitis C virus RNA to individual coagulation proteins, strong interactions between factors XII or XI and RNA was observed, whereas prekallikrein and kininogen showed weaker binding. Other coagulation factors, such as fibrinogen, BSA, and the vitamin K-dependent proteins IX, X, and II (prothrombin), did not bind to RNA (Fig. 3A). Likewise, RNA-complexes containing contact phase proteins were discernable after SDS-gel electrophoresis (SI Fig. 8), which dissappeared after proteinase K treatment. These results indicate that factors XII and XI, prekallikrein and kininogen, can be defined as RNA-binding plasma proteins. When radiolabeled RNA was allowed to react with contact proteins in different stoichiometric ratios followed by electrophoretic separation under nondenaturing conditions, strong interactions between, e.g., factor XI and RNA (Fig. 3B) and kininogen and RNA (data not shown) was noted as reflected by electrophoretic mobility of the complexes. In contrast, no shifting of RNA was seen upon addition of factor X or prothrombin (factor II).
Fig. 3.
Direct binding of nucleic acids to contact phase proteins and protease activation. (A) For detection of detergent-resistent RNA-protein complexes, radiolabeled HCV-RNA and blood coagulation factors as well as BSA were incubated and crosslinked by UV light, followed by RNase treatment, SDS/PAGE on 8% gels, and autoradiography (XII, factor XII; HK, kininogen; PK, prekallikrein; XI, factor XI; IX, factor IX; X, factor X; II, prothrombin; Fbg, fibrinogen). Molecular masses of 14C-labeled marker proteins are indicated along the left margin. (B) For electrophoretic mobility shift assays, radiolabeled HCV-RNA was incubated in the absence or presence of increasing doses (0.1, 0.3, 1, and 3 pmol) each of factor XI, factor X, or factor II, separated on non-denaturing 4% polyacrylamide gels and analyzed by autoradiography. The RNA-protein complexes and the main species of free RNA are indicated, and minor amounts of faster or slower migrating conformers of the unbound RNA are visible. (C) Factor XI alone (open bars), factor XII alone (hatched bars), or both (filled bars) were incubated in a zinc-containing buffer in the absence (−) or presence of RNA or DNA as indicated, and the extent of protease activation was followed by chromogenic substrate assay. (D) Similarly, factor XI alone (open bars), thrombin alone (hatched bars) or both together were analyzed for protease activation in the absence (−) or presence of RNA or DNA as indicated. (E) Shown are different reactions mixtures containing the following: 1, factor XII; 2, prekallikrein; 3, kininogen; 4, factor XII and prekallikrein; 5, factor XII, prekallikrein and kininogen were analyzed for protease activation in the absence (−) or the presence of RNA or DNA. All data represent mean ± SD (n = 3) of a representative experiment of three. (F) The activation of prekallikrein was followed in the presence of increasing doses of tRNA, and enzyme activity was registered by chromogenic substrate cleavage. Note the bell-shaped concentration curve, which is characteristic for a template-related mechanism of RNA as cofactor.
In purified systems, RNA or isolated DNA induced protease activation of factors XII and XI (Fig. 3C) or factor XI and thrombin system (Fig. 3D), the latter representing the essential feedback reaction of coagulation for the production of factor XIa (13). Likewise, in the combination of factor XII, kininogen, and prekallikrein, RNA (or isolated DNA) augmented protease formation by >40-fold compared with control (Fig. 3E). As an example for the concentration-dependent augmentation of protease activation by RNA, kallikrein formation in the presence of kininogen is documented in Fig. 3F. The bell-shaped curve reflects a template mechanism of RNA function that is reminiscent of the molecular mechanism described for high molecular mass heparin in catalyzing thrombin inhibition by antithrombin (25).
Discussion
Under severe conditions of tissue damage, the contribution of the contact phase proteins factors XII and XI in blood coagulation was recently demonstrated in thrombosis models, using factor XII- or factor XI-deficient mice (19, 26, 27), and these data were confirmed here by using an factor XIIa-inhibitor. Under physiological conditions, however, this pathway appears to be dispensable for normal haemostasis. Whereas the mechanisms of contact phase activation under conditions of severe tissue destruction was not addressed in these studies (19, 27), here we provide conclusive evidence that extracellular nucleic acids, particularly RNA, are major cofactors for amplification of blood coagulation. We consider extracellular RNA as our body's “natural foreign surface” to serve as procoagulant cofactor in vivo and as a potential target for the treatment of coagulation-induced thrombosis using RNase. The relationships between vascular injury, nucleic acid exposure, and contact activation remained unconsidered in previous studies (9, 10, 28), although other negatively charged substances such as heparin, sulfatides, or the intracellular heat-shock protein 90 appear to activate contact phase proteins in vitro (11, 29–31). Due to its binding to the forming thrombus in vivo, cell-free RNA may become concentrated at these sites and serves as cofactor for protease activation. Cellular binding sites for factors XII and XI (32, 33) could be shared by RNA, and heparin may compete for factor XII/XI binding to RNA, thereby providing an alternate mechanism of anticoagulation.
The predominant procoagulant role of cell-free polyanionic RNA over DNA particularly in vivo is likely due to the immediate exposure of RNA in the extracellular space after cell damage, whereas the majority of DNA may still be contained in complexed form together with histones in the nuclei and thereby is far less accessible to coagulation factors (34). Moreover, only sufficiently large RNA molecules with a minimal size of ≈50 nucleotides were found to augment enzyme-substrate reactions involving a template-type mechanism, and semiquantative estimation of affinities for RNA-protein binding (derived from mobility shift assay) revealed values in the nM range (15).
Circulating RNase, likely to be derived from endothelial cells (ref. 35; S. Fischer and K.T.P., unpublished data), may balance the functional activity of extracellular RNA under physiological conditions and thereby prevents the contribution of the contact phase in blood coagulation. Another conclusion from this work is the use of RNA-counteracting modalities as potential antithrombotic regimen, because RNase treatment significantly delayed thrombus formation, weakened thrombus structure, and resulted in much earlier recanalization of the occluded site. The antithrombotic effect of RNase was by far more pronounced as compared with the prolongation of occlusion time in plasminogen activator inhibitor-1-deficient animals (21). Thus, on the basis of the extracellular RNA-RNase counteraction described here, a new mechanism of vascular homeostasis is proposed that may contribute in a complementary fashion to other endothelial cell-based regulatory systems (1). It remains to be further established whether additional indirect effects of RNA may contribute to its procoagulant function.
Despite the rapid degradation of RNA, the apparent procoagulant sensitization of plasma or whole blood could contribute to the as yet poorly understood hypercoagulable state observed in patients under thrombotic risk (36). Under conditions of tissue injury in association with sepsis, bacterial or viral infections, the expected increase of extracellular RNA may very well be related to the prothrombotic state in the affected patients. Here, RNase could serve as potent anticoagulant and possibly antiseptic regimen as well. The analysis of circulating RNA or respective RNA-dependent activation products may become a valuable diagnostic tool, and mRNA in plasma of certain cancer patients has been detected after amplification by polymerase-chain-reaction (37).
Finally, the relation between malignancies and a hypercoagulable state or thromboembolic complications in tumor patients has been well documented (17, 38), yet mechanistic clues are pending. Based on our present data, we propose that the increase of extracellular RNA under situations of tumor burden and therapy (17, 18) together with elevated tissue factor (39) could explain many characteristics of a prothrombotic situation, because tumors are “wounds that do not heal” (40). Here, our results could pave the way for understanding the mechanistic relations between the appearance of extracellular RNA and hypercoagulability in malignancy.
Materials and Methods
Materials.
Materials used were as follows: coagulation factors and control proteins (American Diagnostica, Pfungstadt, Germany and Enzyme Research, Essen, Germany); aprotinin (BAYER, Leverkusen, Germany); factor XIIa inhibitor H-d-pro-phe-arg-chloromethylketone (Bachem, Basel, Switzerland); thrombin and corn trypsin inhibitor (Calbiochem, Schwalbach, Germany); hirudin, CJ545, and CJ626 (kind gifts from J. Stuerzebecher, Jena, Germany); recombinant tissue factor and anti-TF 9-6B4 antibody (kindly provided by W. Ruf, La Jolla, CA); chromogenic substrates S-2288, S-2366, and S-2302 (Haemochrom, Essen, Germany); RNase A and DNase I (Fermentas, St. Leon-Rot, Germany); DMEM cell culture medium (GIBCO, Karlsruhe, Germany); yeast tRNA (Sigma, Munich, Germany); coagulation factor-deficient plasmas (DADE–Behring, Liederbach, Germany). Blood samples were taken from healthy volunteers by using 0.38% sodium citrate as anticoagulant and processed within 2 h after withdrawal to obtain platelet-poor-plasma. Aliquots were snapfrozen until further use. Cellular nucleic acids (total cellular RNA or genomic DNA) were isolated from cultured Chinese hamster ovary (CHO) cells or yeast, E. coli, and Qβ-phage by using TRIzol-reagent (Invitrogen, Karlsruhe, Germany), an RNeasy kit, or a genomic DNA isolation kit (Qiagen, Hilden, Germany).
Cell Lysate.
Cell lysate from 50 × 106 CHO cells was generated by exposing washed cells to three freeze-thaw cycles in 1 ml 0.1 M imidazol, pH 7.4, between liquid nitrogen and a 37°C water bath. Cell lysates were cleared by centrifugation (22,000 × g for 15 min), and their procoagulant activity was analyzed in an turbidity assay. In BSA-blocked microtiter plate wells, 30-μl cell lysate dilutions were pretreated with each 10 units per ml of RNase A or DNase I, 10 μg/ml of anti-TF9-6B4 in 0.1 M imidazol buffer, pH 7.4, for 30 min at 37°C. After the addition of 30 μl of normal human plasma, coagulation was initiated after 5 min by recalcification with 100 μl of 20 mM CaCl2-solution.
Serine Protease Activity in Plasma.
After blocking microtiter plate wells with 3% BSA solution, 10 μl of normal human plasma in a total volume of 100 μl 0.1 M imidazol buffer, pH 7.4, containing 0.2 mM chromogenic substrate S-2288 was incubated in the absence or presence of 10 μg/ml of cellular total RNA or genomic DNA, hydrolyzed RNA, or DNA. As controls, 10 ng/ml of kaolin or 0.8 μg/ml of recombinant tissue factor were added instead of nucleic acids. For inhibitors studies, serial dilutions of aprotinin, corn trypsin inhibitor, hirudin, CJ545, or CJ626 were included, and after addition of 30 μg/ml of total cellular RNA, the reaction was started by the addition of citrated plasma. Absorbance at 405 nm was measured up to 60 min, and Vmax was determined by KC4 software (BIO-TEK, Bad Friedrichshall, Germany).
Reconstitution of Contact Phase Pathway.
Isolated coagulation proteins dissolved in 150 mM NaCl/10 mM Tris·HCl, pH 7.4 were added alone or in combinations (see Fig. 3 legend) to preblocked (3% BSA) microtiter wells, and protease activity in the absence or presence of 1 μg/ml each of cellular RNA or genomic DNA was recorded in the presence of 0.2 mM of chromogenic substrate S-2366. Either 50 μM ZnCl2 or 2 mM CaCl2 was present in the reaction mixtures. To evaluate the template effect of RNA, 20 μM prekallikrein was incubated with 20 nM high molecular weight kininogen in the presence of increasing concentrations of yeast total RNA and 0.3 mM chromogenic substrate S-2302 and 50 μM ZnCl2. Protease activity was recorded at 405 nm by using KC4 software (BIO-TEK).
FeCl3-Induced Arterial Thrombosis.
Wild-type C57BL6/J mice were anesthetized by intraperitoneal injection with imidazolame [5 mg/kg body weight (BW); Ratiopharm, Ulm, Germany], medetomidine (0.5 mg/kg BW; Pfizer, Karlsruhe, Germany), and fentanyl (0.05 mg/kg BW; CuraMed Pharma, Munich, Germany). Polyethylene catheters (Portex, Hythe, U. K.) were implanted into the right jugular vein, and subsequently, RNase A or DNase (100 μl each of a 1 mg/ml solution containing 0.9% NaCl, heat-pretreated to destroy protease activity), factor XIIa inhibitor (10 mg/kg BW) (27), or vehicle (0.9% NaCl) were administered i.v. via the jugular vein. Platelets (60 × 106 platelets) were isolated from separate donor animals and labeled with 5-carboxyfluorescein diacetate succinimidyl ester as described earlier (41) and infused i.v. Finally, the right common carotid artery was dissected free and vascular injury of the carotid artery was induced by local application of ferric chloride (FeCl3) essentially as described in ref. 21. In brief, a filter paper (0.5 × 1.0 mm) saturated with 10% FeCl3 was applied to the adventitial surface of the vessel. Arterial thrombosis was monitored by intravital videofluorescence microscopy of fluorescence-tagged platelets (21).
To visualize thrombus-bound RNA, a 25 μM stock solution of SYTO RNASelect green fluorescent cell stain (Molecular Probes, Poortgebouw, The Netherlands) was freshly prepared, and immediately before FeCl3 application, 60 μl of this solution was injected into the left jugular vein. In the treatment group, 15 μg of RNase A was dissolved in 50 μl of 0.9% NaCl and administered via tail vein injection 30 min and immediately before the described thrombus induction by FeCl3 treatment. In the control group, the same amount of 0.9% NaCl was administered. RNase A was incubated at 95°C for 15 min to inactivate any decontaminating proteases before administration. Fifteen minutes after FeCl3 administration, arteries were clamped, and the mice were killed with an overdose of the anesthetic. Arteries were excised and embedded in Tissue Tek OCT (Miles Laboratories, Naperville, IL), snap-frozen, and stored at −80°C until use. Samples were sectioned on a Leica cryostat (6 μm) and placed on slides coated with poly(l-lysine) (Sigma) for immunohistochemical analysis. For morphometric analyses, hematoxylin and eosin staining was performed according to standard protocols. After blocking sections with 10% normal goat serum, platelets were detected by using monoclonal rat anti-mouse CD41 (glycoprotein Ib, 1:200; BD Biosciences, Heidelberg, Germany) (42), and goat anti-rat IgG conjugated to Alexa Fluor 546 (Molecular Probes). Slides were mounted in Vectashield medium H-1000, containing DAPI, 2HCl (Calbiochem), and evaluated by using an epifluorescence microscope (DMRB; Leica, Wetzlar, Germany). All procedures involving experimental animals were approved by the institutional committee for animal research of the Technical University (Munich) and the Justus-Liebig-University (Giessen) and complied with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 86-23, revised 1985).
Statistical Analysis.
Unless otherwise stated, all data are presented as mean ± SEM. All statistics were performed by using SPSS software; siginificance level was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank Christine Moosbauer, Thomas Schmidt-Wöll, Uwe Schubert, and Kathrin Petri for their assistance, Dr. Christof Biebricher (Max-Planck-Institute, Göttingen, Germany) for his generous gift of Qβ-phage RNA, Dr. Takatoshi Koyama and Prof. Ryuichi Kamiyama (Tokyo, Japan), Dr. Sebastian Schmidt (Giessen, Germany), and Drs. Jürgen Römisch and Thomas Weimer (Marburg, Germany). This work was funded by Deutsche Forschungsgemeinschaft (Bonn, Germany) Grants SFB-547; SFB-535; GRK-370; KFO-118; SPP-1190, Aventis Behring (Marburg, Germany), and the German–Israeli Foundation (Jerusalem, Israel).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608647104/DC1.
References
- 1.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmermann GA, McEver RP, Pober JS, Wicj TM, Konkle BA, Schwartz BS, et al. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 2.Ruf W, Mueller BM. Thromb Haemost. 1999;82:175–182. [PubMed] [Google Scholar]
- 3.Mann KG, Butenas S, Brummel K. Arterioscler Thromb Vasc Biol. 2003;23:17–25. doi: 10.1161/01.atv.0000046238.23903.fc. [DOI] [PubMed] [Google Scholar]
- 4.Müller I, Klocke A, Alex M, Kotzsch M, Luther T, Morgenstern E, Zieseniss S, Zahler S, Preissner KT, Engelmann B. FASEB J. 2003;17:476–478. doi: 10.1096/fj.02-0574fje. [DOI] [PubMed] [Google Scholar]
- 5.Davie EW, Ratnoff OD. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 6.MacFarlane RG. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 7.Dahlbäck B. Lancet. 2000;355:1627–1632. doi: 10.1016/S0140-6736(00)02225-X. [DOI] [PubMed] [Google Scholar]
- 8.Colman RW, Schmaier AH. Blood. 1997;90:3819–3843. [PubMed] [Google Scholar]
- 9.Ratnoff OD, Saito H. Curr Top Hematol. 1979;2:1–57. [PubMed] [Google Scholar]
- 10.Cochrane CG, Griffin JH. Adv Immunol. 1982;33:241–306. doi: 10.1016/s0065-2776(08)60837-8. [DOI] [PubMed] [Google Scholar]
- 11.Hojima Y, Cochrane CG, Wiggins RC, Austen KF, Stevens RL. Blood. 1984;63:1453–1459. [PubMed] [Google Scholar]
- 12.Kitchens CS. Arch Pathol Lab Med. 2002;126:1382–1386. doi: 10.5858/2002-126-1382-TCS. [DOI] [PubMed] [Google Scholar]
- 13.Gailani D, Broze GJ. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 14.Gailani D, Lasky NM, Broze GJ. Blood Coagul Fibrinol. 1997;8:134–144. doi: 10.1097/00001721-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Nakazawa F, Kannemeier C, Shibamiya A, Song Y, Tzima E, Schubert U, Koyama T, Niepmann M, Trusheim H, Engelmann B, Preissner KT. Biochem J. 2005;385:831–838. doi: 10.1042/BJ20041021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altincicek B, Shibamiya A, Trusheim H, Tzima E, Niepmann M, Linder D, Preissner KT, Kanse SM. Biochem J. 2006;394:687–692. doi: 10.1042/BJ20051563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Olmo DC, Ruiz-Piqueras R, Garcia-Olmo D. Histol Histopathol. 2004;19:575–583. doi: 10.14670/HH-19.575. [DOI] [PubMed] [Google Scholar]
- 18.Wieczorek AJ, Rhyner C, Block LH. Proc Natl Acad Sci USA. 1985;82:3455–3459. doi: 10.1073/pnas.82.10.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renné T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni H, Ramakrishnan V, Ruggeri ZM, Papalia JM, Phillips DR, Wagner DD. Blood. 2001;98:368–373. doi: 10.1182/blood.v98.2.368. [DOI] [PubMed] [Google Scholar]
- 21.Konstantinides S, Schäfer K, Thinnes T, Loskutoff DJ. Circulation. 2001;103:576–583. doi: 10.1161/01.cir.103.4.576. [DOI] [PubMed] [Google Scholar]
- 22.Fay WP, Parker AC, Ansari MN, Zheng X, Ginsburg D. Blood. 1999;93:1825–1830. [PubMed] [Google Scholar]
- 23.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 24.Proctor RR, Rapaport SI. Am J Clin Pathol. 1961;36:212–219. doi: 10.1093/ajcp/36.3.212. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Johnson DJ, Esmon CT, Huntington JA. Nat Struct Mol Biol. 2004;11:857–862. doi: 10.1038/nsmb811. [DOI] [PubMed] [Google Scholar]
- 26.Rosen ED, Gailani D, Castellino FJ. Thromb Haemost. 2002;87:774–776. [PubMed] [Google Scholar]
- 27.Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, Renné C, Gailani D, Nieswandt B, Renné T. J Exp Med. 2006;203:513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmaier AH. Thromb Haemost. 1997;78:101–107. [PubMed] [Google Scholar]
- 29.Joseph K, Tholanikunnel BG, Kaplan AP. Proc Natl Acad Sci USA. 2002;99:896–900. doi: 10.1073/pnas.022626899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espana F, Ratnoff OD. J Lab Clin Med. 1983;102:487–499. [PubMed] [Google Scholar]
- 31.Tans G, Rosing J, Griffin JH. J Biol Chem. 1983;258:8215–8222. [PubMed] [Google Scholar]
- 32.Walsh PN, Griffin JH. Blood. 1981;57:106–118. [PubMed] [Google Scholar]
- 33.Ho DH, Badellino K, Baglia FA, Walsh PN. J Biol Chem. 1998;273:16382–16390. doi: 10.1074/jbc.273.26.16382. [DOI] [PubMed] [Google Scholar]
- 34.Galeazzi M, Morozzi G, Piccini M, Chen J, Bellisai F, Fineschi S, Marcolongo R. Autoimmun Rev. 2003;2:50–55. doi: 10.1016/s1568-9972(02)00101-5. [DOI] [PubMed] [Google Scholar]
- 35.Landré JB, Hewett PW, Olivot JM, Friedl P, Ko Y, Sachinidis A, Moenner M. J Cell Biochem. 2002;86:540–552. doi: 10.1002/jcb.10234. [DOI] [PubMed] [Google Scholar]
- 36.Meissner MH, Chandler WL, Elliott JS. J Trauma. 2003;54:224–231. doi: 10.1097/01.TA.0000046253.33495.70. [DOI] [PubMed] [Google Scholar]
- 37.Ng EK, Tsui NB, Lam NY, Chiu RW, Yu SC, Wong SC, Lo ES, Rainer TH, Johnson PJ, Lo YM. Clin Chem. 2002;48:1212–1217. [PubMed] [Google Scholar]
- 38.Sorensen HT, Johson SP, Norgard B, Zacharski LR, Baron JA. Clin Lab. 2003;49:615–623. [PubMed] [Google Scholar]
- 39.Zhang Y, Deng Y, Luther T, Muller M, Ziegler R, Waldherr R, Stern DM, Nawroth PP. J Clin Invest. 1994;94:1320–1327. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dvorak HF. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 41.Massberg S, Gawaz M, Gruner S, Schulte V, Konrad I, Zohlnhofer D, Heinzmann U, Nieswandt BA. J Exp Med. 2003;197:41–49. doi: 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieswandt B, Echtenacher B, Wachs FP, Schröder J, Gessner JE, Schmidt RE, Grau GE, Maennel DN. Blood. 1999;94:684–693. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.